автордың кітабын онлайн тегін оқу On Growth and Form
GROWTH AND FORM
CAMBRIDGE UNIVERSITY PRESS
C. F. CLAY, MANAGER
London: FETTER LANE, E.C.
Edinburgh: 100 PRINCES STREET

New York: G. P. PUTNAM’S SONS
Bombay, Calcutta and Madras: MACMILLAN AND Co., LTD.
Toronto: J. M. DENT AND SONS, LTD.
Tokyo: THE MARUZEN-KABUSHIKI-KAISHA
All rights reserved
ON
GROWTH AND FORM
BY
D’ARCY WENTWORTH THOMPSON
Cambridge:
at the University Press
1917
“The reasonings about the wonderful and intricate operations of nature are so full of uncertainty, that, as the Wise-man truly observes, hardly do we guess aright at the things that are upon earth, and with labour do we find the things that are before us.” Stephen Hales, Vegetable Staticks (1727), p. 318, 1738.
PREFATORY NOTE
This book of mine has little need of preface, for indeed it is “all preface” from beginning to end. I have written it as an easy introduction to the study of organic Form, by methods which are the common-places of physical science, which are by no means novel in their application to natural history, but which nevertheless naturalists are little accustomed to employ.
It is not the biologist with an inkling of mathematics, but the skilled and learned mathematician who must ultimately deal with such problems as are merely sketched and adumbrated here. I pretend to no mathematical skill, but I have made what use I could of what tools I had; I have dealt with simple cases, and the mathematical methods which I have introduced are of the easiest and simplest kind. Elementary as they are, my book has not been written without the help—the indispensable help—of many friends. Like Mr Pope translating Homer, when I felt myself deficient I sought assistance! And the experience which Johnson attributed to Pope has been mine also, that men of learning did not refuse to help me.
My debts are many, and I will not try to proclaim them all: but I beg to record my particular obligations to Professor Claxton Fidler, Sir George Greenhill, Sir Joseph Larmor, and Professor A. McKenzie; to a much younger but very helpful friend, Mr John Marshall, Scholar of Trinity; lastly, and (if I may say so) most of all, to my colleague Professor William Peddie, whose advice has made many useful additions to my book and whose criticism has spared me many a fault and blunder.
I am under obligations also to the authors and publishers of many books from which illustrations have been borrowed, and especially to the following:—
To the Controller of H.M. Stationery Office, for leave to reproduce a number of figures, chiefly of Foraminifera and of Radiolaria, from the Reports of the Challenger Expedition. {vi}
To the Council of the Royal Society of Edinburgh, and to that of the Zoological Society of London:—the former for letting me reprint from their Transactions the greater part of the text and illustrations of my concluding chapter, the latter for the use of a number of figures for my chapter on Horns.
To Professor E. B. Wilson, for his well-known and all but indispensable figures of the cell (figs. 42–51, 53); to M. A. Prenant, for other figures (41, 48) in the same chapter; to Sir Donald MacAlister and Mr Edwin Arnold for certain figures (335–7), and to Sir Edward Schäfer and Messrs Longmans for another (334), illustrating the minute trabecular structure of bone. To Mr Gerhard Heilmann, of Copenhagen, for his beautiful diagrams (figs. 388–93, 401, 402) included in my last chapter. To Professor Claxton Fidler and to Messrs Griffin, for letting me use, with more or less modification or simplification, a number of illustrations (figs. 339–346) from Professor Fidler’s Textbook of Bridge Construction. To Messrs Blackwood and Sons, for several cuts (figs. 127–9, 131, 173) from Professor Alleyne Nicholson’s Palaeontology; to Mr Heinemann, for certain figures (57, 122, 123, 205) from Dr Stéphane Leduc’s Mechanism of Life; to Mr A. M. Worthington and to Messrs Longmans, for figures (71, 75) from A Study of Splashes, and to Mr C. R. Darling and to Messrs E. and S. Spon for those (fig. 85) from Mr Darling’s Liquid Drops and Globules. To Messrs Macmillan and Co. for two figures (304, 305) from Zittel’s Palaeontology, to the Oxford University Press for a diagram (fig. 28) from Mr J. W. Jenkinson’s Experimental Embryology; and to the Cambridge University Press for a number of figures from Professor Henry Woods’s Invertebrate Palaeontology, for one (fig. 210) from Dr Willey’s Zoological Results, and for another (fig. 321) from “Thomson and Tait.”
Many more, and by much the greater part of my diagrams, I owe to the untiring help of Dr Doris L. Mackinnon, D.Sc., and of Miss Helen Ogilvie, M.A., B.Sc., of this College.
D’ARCY WENTWORTH THOMPSON.
UNIVERSITY COLLEGE, DUNDEE.
December, 1916.
Fig. 42.
Fig. 43.
Fig. 50.
Fig. 51.
Fig. 53. Annular chromosomes, formed in the spermatogenesis of the Mole-cricket. (From Wilson, after Vom Rath.)
Fig. 41. Caryokinetic figure in a dividing cell (or blastomere) of the Trout’s egg. (After Prenant, from a preparation by Prof. P. Bouin.)
Fig. 48.
Fig. 49.
Fig. 335. Crane-head and femur. (After Culmann and H. Meyer.)
Fig. 337. Trabecular structure of the os calcis. (From MacAlister.)
Fig. 334. Head of the human femur in section. (After Schäfer, from a photo by Prof. A. Robinson.)
Fig. 388. Pelvis of Archaeopteryx.
Fig. 393. The pelves of Archaeopteryx and of Apatornis, with three transitional types interpolated between them.
Fig. 401. A, outline diagram of the Cartesian co-ordinates of the skull of Hyracotherium or Eohippus, as shewn in Fig. 402, A. H, outline of the corresponding projection of the horse’s skull. B–G, intermediate, or interpolated, outlines.
Fig. 402. A, skull of Hyracotherium, from the Eocene, after W. B. Scott; H, skull of horse, represented as a co-ordinate transformation of that of Hyracotherium, and to the same scale of magnitude; B–G, various artificial or imaginary types, reconstructed as intermediate stages between A and H; M, skull of Mesohippus, from the Oligocene, after Scott, for comparison with C; P, skull of Protohippus, from the Miocene, after Cope, for comparison with E; Pp, lower jaw of Protohippus placidus (after Matthew and Gidley), for comparison with F; Mi, Miohippus (after Osborn), Pa, Parahippus (after Peterson), shewing resemblance, but less perfect agreement, with C and D.
Fig. 339.
Fig. 346.
Fig. 127.
Lithostrotion Martini.(After Nicholson.)
Fig. 128.
Cyathophyllum hexagonum.(From Nicholson, after Zittel.)
Fig. 9. Curve of pre-natal growth (length or stature) of child; and corresponding curve of mean monthly increments (mm.).
Fig. 131. Surface-views of Corals with undeveloped thecae and confluent septa. A, Thamnastraea; B, Comoseris. (From Nicholson, after Zittel.)
Fig. 173. Heterophyllia angulata. (After Nicholson.)
Fig. 57. Artificial caryokinesis (after Leduc), for comparison with Fig. 41, p. 169.
Fig. 122. An “artificial tissue,” formed by coloured drops of sodium chloride solution diffusing in a less dense solution of the same salt. (After Leduc.)
Fig. 123. An artificial cellular tissue, formed by the diffusion in gelatine of drops of a solution of potassium ferrocyanide. (After Leduc.)
Fig. 205. Liesegang’s Rings. (After Leduc.)
Fig. 71. A breaking wave. (From Worthington.)
Fig. 75. Various species of Vorticella. (Mostly after Saville Kent.)
Fig. 85. (After Darling.)
Fig. 304. Section of Nautilus, shewing the contour of the septa in the median plane: the septa being (in this plane) logarithmic spirals, of which the shell-spiral is the evolute.
Fig. 305. Cast of the interior of Nautilus: to shew the contours of the septa at their junction with the shell-wall.
Fig. 28. Diagram shewing time taken (in days), at various temperatures (°C.), to reach certain stages of development in the Frog: viz. I, gastrula; II, medullary plate; III, closure of medullary folds; IV, tail-bud; V, tail and gills; VI, tail-fin; VII, operculum beginning; VIII, do. closing; IX, first appearance of hind-legs. (From Jenkinson, after O. Hertwig, 1898.)
Fig. 210. Close-packed calcospherites, or so-called “spicules,” of Astrosclera. (After Lister.)
Fig. 321.
Fig. 322.
CONTENTS
CHAP.
PAGE
I.
INTRODUCTORY 1II.
ON MAGNITUDE 16III.
THE RATE OF GROWTH 50IV.
ON THE INTERNAL FORM AND STRUCTURE OF THE CELL 156V.
THE FORMS OF CELLS 201VI.
A
NOTE ON ADSORPTION 277VII.
THE FORMS OF TISSUES, OR CELL-AGGREGATES 293VIII.
THE SAME(
continued)
346IX.
ON CONCRETIONS, SPICULES, AND SPICULAR SKELETONS 411X.
A
PARENTHETIC NOTE ON GEODETICS 488XI.
THE LOGARITHMIC SPIRAL 493XII.
THE SPIRAL SHELLS OF THE FORAMINIFERA 587XIII.
THE SHAPES OF HORNS, AND OF TEETH OR TUSKS: WITH A NOTE ON TORSION 612XIV.
ON LEAF-ARRANGEMENT, OR PHYLLOTAXIS 635XV.
ON THE SHAPES OF EGGS, AND OF CERTAIN OTHER HOLLOW STRUCTURES 652XVI.
ON FORM AND MECHANICAL EFFICIENCY 670XVII.
ON THE THEORY OF TRANSFORMATIONS, OR THE COMPARISON OF RELATED FORMS 719 EPILOGUE 778 INDEX 780CHAPTER I INTRODUCTORY
CHAPTER II. ON MAGNITUDE
CHAPTER III THE RATE OF GROWTH
CHAPTER IVON THE INTERNAL FORM AND STRUCTURE OF THE CELL
CHAPTER V THE FORMS OF CELLS
CHAPTER VI A NOTE ON ADSORPTION
CHAPTER VII THE FORMS OF TISSUES OR CELL-AGGREGATES
CHAPTER VIII THE FORMS OF TISSUES OR CELL-AGGREGATES (continued)
CHAPTER IX ON CONCRETIONS, SPICULES, AND SPICULAR SKELETONS
CHAPTER X A PARENTHETIC NOTE ON GEODETICS
CHAPTER XI THE LOGARITHMIC SPIRAL
CHAPTER XII THE SPIRAL SHELLS OF THE FORAMINIFERA
CHAPTER XIII THE SHAPES OF HORNS, AND OF TEETH OR TUSKS: WITH A NOTE ON TORSION
CHAPTER XIV ON LEAF-ARRANGEMENT, OR PHYLLOTAXIS
CHAPTER XV ON THE SHAPES OF EGGS, AND OF CERTAIN OTHER HOLLOW STRUCTURES
CHAPTER XVI ON FORM AND MECHANICAL EFFICIENCY
CHAPTER XVII ON THE THEORY OF TRANSFORMATIONS, OR THE COMPARISON OF RELATED FORMS*
EPILOGUE.
INDEX.
LIST OF ILLUSTRATIONS
Fig.
Page
1.
Nerve-cells, from larger and smaller animals (Minot, after Irving Hardesty)
37
2.
Relative magnitudes of some minute organisms (Zsigmondy)
39
3.
Curves of growth in man (Quetelet and Bowditch)
61
4, 5.
Mean annual increments of stature and weight in man (
do.)
66, 69
6.
The ratio, throughout life, of female weight to male (
do.)
71
7–9.
Curves of growth of child, before and after birth (His and Rüssow)
74–6
10.
Curve of growth of bamboo (Ostwald, after Kraus)
77
11.
Coefficients of variability in human stature (Boas and Wissler)
80
12.
Growth in weight of mouse (Wolfgang Ostwald)
83
13.
Do.of silkworm (Luciani and Lo Monaco)
84
14.
Do.of tadpole (Ostwald, after Schaper)
85
15.
Larval eels, or
Leptocephali, and young elver (Joh. Schmidt)
86
16.
Growth in length of
Spirogyra(Hofmeister)
87
17.
Pulsations of growth in
Crocus(Bose)
88
18.
Relative growth of brain, heart and body of man (Quetelet)
90
19.
Ratio of stature to span of arms (
do.)
94
20.
Rates of growth near the tip of a bean-root (Sachs)
96
21, 22.
The weight-length ratio of the plaice, and its annual periodic changes
99, 100
23.
Variability of tail-forceps in earwigs (Bateson)
104
24.
Variability of body-length in plaice
105
25.
Rate of growth in plants in relation to temperature (Sachs)
109
26.
Do.in maize, observed (Köppen), and calculated curves
112
27.
Do.in roots of peas (Miss I. Leitch)
113
28, 29.
Rate of growth of frog in relation to temperature (Jenkinson, after O. Hertwig), and calculated curves of
do.115, 6
30.
Seasonal fluctuation of rate of growth in man (Daffner)
119
31.
Do.in the rate of growth of trees (C. E. Hall)
120
32.
Long-period fluctuation in the rate of growth of Arizona trees (A. E. Douglass)
122
33, 34.
The varying form of brine-shrimps (
Artemia), in relation to salinity (Abonyi)
128, 9
35–39.
Curves of regenerative growth in tadpoles’ tails (M. L. Durbin)
140–145
40.
Relation between amount of tail removed, amount restored, and time required for restoration (M. M. Ellis)
148
41.
Caryokinesis in trout’s egg (Prenant, after Prof. P. Bouin)
169
42–51.
Diagrams of mitotic cell-division (Prof. E. B. Wilson)
171–5
52.
Chromosomes in course of splitting and separation (Hatschek and Flemming)
180
53.
Annular chromosomes of mole-cricket (Wilson, after vom Rath)
181
54–56.
Diagrams illustrating a hypothetic field of force in caryokinesis (Prof. W. Peddie)
182–4
57.
An artificial figure of caryokinesis (Leduc)
186
58.
A segmented egg of
Cerebratulus(Prenant, after Coe)
189
59.
Diagram of a field of force with two like poles
189
60.
A budding yeast-cell
213
61.
The roulettes of the conic sections
218
62.
Mode of development of an unduloid from a cylindrical tube
220
63–65.
Cylindrical, unduloid, nodoid and catenoid oil-globules (Plateau)
222, 3
66.
Diagram of the nodoid, or elastic curve
224
67.
Diagram of a cylinder capped by the corresponding portion of a sphere
226
68.
A liquid cylinder breaking up into spheres
227
69.
The same phenomenon in a protoplasmic cell of
Trianea234
70.
Some phases of a splash (A. M. Worthington)
235
71.
A breaking wave (
do.)
236
72.
The calycles of some campanularian zoophytes
237
73.
A flagellate monad,
Distigma proteus(Saville Kent)
246
74.
Noctiluca miliaris, diagrammatic
246
75.
Various species of
Vorticella(Saville Kent and others)
247
76.
Various species of
Salpingoeca(
do.)
248
77.
Species of
Tintinnus,
Dinobryonand
Codonella(
do.)
248
78.
The tube or cup of
Vaginicola248
79.
The same of
Folliculina249
80.
Trachelophyllum(Wreszniowski)
249
81.
Trichodina pediculus
252
82.
Dinenymplia gracilis(Leidy)
253
83.
A “collar-cell” of
Codosiga254
84.
Various species of
Lagena(Brady)
256
85.
Hanging drops, to illustrate the unduloid form (C. R. Darling)
257
86.
Diagram of a fluted cylinder
260
87.
Nodosaria scalaris(Brady)
262
88.
Fluted and pleated gonangia of certain Campanularians (Allman)
262
89.
Various species of
Nodosaria,
Sagrinaand
Rheophax(Brady)
263
90.
Trypanosoma tineaeand
Spirochaeta anodontae, to shew undulating membranes (Minchin and Fantham)
266
91.
Some species of
Trichomastixand
Trichomonas(Kofoid)
267
92.
Herpetomonasassuming the undulatory membrane of a Trypanosome (D. L. Mackinnon)
268
93.
Diagram of a human blood-corpuscle
271
94.
Sperm-cells of decapod crustacea,
Inachusand
Galathea(Koltzoff)
273
95.
The same, in saline solutions of varying density (
do.)
274
96.
A sperm-cell of
Dromia(
do.)
275
97.
Chondriosomes in cells of kidney and pancreas (Barratt and Mathews)
285
98.
Adsorptive concentration of potassium salts in various plant-cells (Macallum)
290
99–101.
Equilibrium of surface-tension in a floating drop
294, 5
102.
Plateau’s “bourrelet” in plant-cells; diagrammatic (Berthold)
298
103.
Parenchyma of maize, shewing the same phenomenon
298
104, 5.
Diagrams of the partition-wall between two soap-bubbles
299, 300
106.
Diagram of a partition in a conical cell
300
107.
Chains of cells in
Nostoc,
Anabaenaand other low algae
300
108.
Diagram of a symmetrically divided soap-bubble
301
109.
Arrangement of partitions in dividing spores of
Pellia(Campbell)
302
110.
Cells of
Dictyota(Reinke)
303
111, 2.
Terminal and other cells of
Chara, and young antheridium of
do.303
113.
Diagram of cell-walls and partitions under various conditions of tension
304
114, 5.
The partition-surfaces of three interconnected bubbles
307, 8
116.
Diagram of four interconnected cells or bubbles
309
117.
Various configurations of four cells in a frog’s egg (Rauber)
311
118.
Another diagram of two conjoined soap-bubbles
313
119.
A froth of bubbles, shewing its outer or “epidermal” layer
314
120.
A tetrahedron, or tetrahedral system, shewing its centre of symmetry
317
121.
A group of hexagonal cells (Bonanni)
319
122, 3.
Artificial cellular tissues (Leduc)
320
124.
Epidermis of
Girardia(Goebel)
321
125.
Soap-froth, and the same under compression (Rhumbler)
322
126.
Epidermal cells of
Elodea canadensis(Berthold)
322
127.
Lithostrotion Martini(Nicholson)
325
128.
Cyathophyllum hexagonum(Nicholson, after Zittel)
325
129.
Arachnophyllum pentagonum(Nicholson)
326
130.
Heliolites(Woods)
326
131.
Confluent septa in
Thamnastraeaand
Comoseris(Nicholson, after Zittel)
327
132.
Geometrical construction of a bee’s cell
330
133.
Stellate cells in the pith of a rush; diagrammatic
335
134.
Diagram of soap-films formed in a cubical wire skeleton (Plateau)
337
135.
Polar furrows in systems of four soap-bubbles (Robert)
341
136–8.
Diagrams illustrating the division of a cube by partitions of minimal area
347–50
139.
Cells from hairs of
Sphacelaria(Berthold)
351
140.
The bisection of an isosceles triangle by minimal partitions
353
141.
The similar partitioning of spheroidal and conical cells
353
142.
S-shaped partitions from cells of algae and mosses (Reinke and others)
355
143.
Diagrammatic explanation of the S-shaped partitions
356
144.
Development of
Erythrotrichia(Berthold)
359
145.
Periclinal, anticlinal and radial partitioning of a quadrant
359
146.
Construction for the minimal partitioning of a quadrant
361
147.
Another diagram of anticlinal and periclinal partitions
362
148.
Mode of segmentation of an artificially flattened frog’s egg (Roux)
363
149.
The bisection, by minimal partitions, of a prism of small angle
364
150.
Comparative diagram of the various modes of bisection of a prismatic sector
365
151.
Diagram of the further growth of the two halves of a quadrantal cell
367
152.
Diagram of the origin of an epidermic layer of cells
370
153.
A discoidal cell dividing into octants
371
154.
A germinating spore of
Riccia(after Campbell), to shew the manner of space-partitioning in the cellular tissue
372
155, 6.
Theoretical arrangement of successive partitions in a discoidal cell
373
157.
Sections of a moss-embryo (Kienitz-Gerloff)
374
158.
Various possible arrangements of partitions in groups of four to eight cells
375
159.
Three modes of partitioning in a system of six cells
376
160, 1.
Segmenting eggs of
Trochus(Robert), and of
Cynthia(Conklin)
377
162.
Section of the apical cone of
Salvinia(Pringsheim)
377
163, 4.
Segmenting eggs of
Pyrosoma(Korotneff), and of
Echinus(Driesch)
377
165.
Segmenting egg of a cephalopod (Watase)
378
166, 7.
Eggs segmenting under pressure: of
Echinusand
Nereis(Driesch), and of a frog (Roux)
378
168.
Various arrangements of a group of eight cells on the surface of a frog’s egg (Rauber)
381
169.
Diagram of the partitions and interfacial contacts in a system of eight cells
383
170.
Various modes of aggregation of eight oil-drops (Roux)
384
171.
Forms, or species, of
Asterolampra(Greville)
386
172.
Diagrammatic section of an alcyonarian polype
387
173, 4.
Sections of
Heterophyllia(Nicholson and Martin Duncan)
388, 9
175.
Diagrammatic section of a ctenophore (
Eucharis)
391
176, 7.
Diagrams of the construction of a Pluteus larva
392, 3
178, 9.
Diagrams of the development of stomata, in
Sedumand in the hyacinth
394
180.
Various spores and pollen-grains (Berthold and others)
396
181.
Spore of
Anthoceros(Campbell)
397
182, 4, 9.
Diagrammatic modes of division of a cell under certain conditions of asymmetry
400–5
183.
Development of the embryo of
Sphagnum(Campbell)
402
185.
The gemma of a moss (
do.)
403
186.
The antheridium of
Riccia(
do.)
404
187.
Section of growing shoot of
Selaginella, diagrammatic
404
188.
An embryo of
Jungermannia(Kienitz-Gerloff)
404
190.
Development of the sporangium of
Osmunda(Bower)
406
191.
Embryos of
Phascumand of
Adiantum(Kienitz-Gerloff)
408
192.
A section of
Girardia(Goebel)
408
193.
An antheridium of
Pteris(Strasburger)
409
194.
Spicules of
Siphonogorgiaand
Anthogorgia(Studer)
413
195–7.
Calcospherites, deposited in white of egg (Harting)
421, 2
198.
Sections of the shell of
Mya(Carpenter)
422
199.
Concretions, or spicules, artificially deposited in cartilage (Harting)
423
200.
Further illustrations of alcyonarian spicules:
Eunicea(Studer)
424
201–3.
Associated, aggregated and composite calcospherites (Harting)
425, 6
204.
Harting’s “conostats”
427
205.
Liesegang’s rings (Leduc)
428
206.
Relay-crystals of common salt (Bowman)
429
207.
Wheel-like crystals in a colloid medium (
do.)
429
208.
A concentrically striated calcospherite or spherocrystal (Harting)
432
209.
Otoliths of plaice, shewing “age-rings” (Wallace)
432
210.
Spicules, or calcospherites, of
Astrosclera(Lister)
436
211. 2.
C- and S-shaped spicules of sponges and holothurians (Sollas and Théel)
442
213.
An amphidisc of
Hyalonema442
214–7.
Spicules of calcareous, tetractinellid and hexactinellid sponges, and of various holothurians (Haeckel, Schultze, Sollas and Théel)
445–452
218.
Diagram of a solid body confined by surface-energy to a liquid boundary-film
460
219.
Astrorhiza limicolaand
arenaria(Brady)
464
220.
A nuclear “
reticulum plasmatique” (Carnoy)
468
221.
A spherical radiolarian,
Aulonia hexagona(Haeckel)
469
222.
Actinomma arcadophorum(
do.)
469
223.
Ethmosphaera conosiphonia(
do.)
470
224.
Portions of shells of
Cenosphaera favosaand
vesparia(
do.)
470
225.
Aulastrum triceros(
do.)
471
226.
Part of the skeleton of
Cannorhaphis(
do.)
472
227.
A Nassellarian skeleton,
Callimitra carolotae(
do.)
472
228, 9.
Portions of
Dictyocha stapedia(
do.)
474
230.
Diagram to illustrate the conformation of
Callimitra476
231.
Skeletons of various radiolarians (Haeckel)
479
232.
Diagrammatic structure of the skeleton of
Dorataspis(
do.)
481
233, 4.
Phatnaspis cristata(Haeckel), and a diagram of the same
483
235.
Phractaspis prototypus(Haeckel)
484
236.
Annular and spiral thickenings in the walls of plant-cells
488
237.
A radiograph of the shell of
Nautilus(Green and Gardiner)
494
238.
A spiral foraminifer,
Globigerina(Brady)
495
239–42.
Diagrams to illustrate the development or growth of a logarithmic spiral
407–501
243.
A helicoid and a scorpioid cyme
502
244.
An Archimedean spiral
503
245–7.
More diagrams of the development of a logarithmic spiral
505, 6
248–57.
Various diagrams illustrating the mathematical theory of gnomons
508–13
258.
A shell of
Haliotis, to shew how each increment of the shell constitutes a gnomon to the preexisting structure
514
259, 60.
Spiral foraminifera,
Pulvinulinaand
Cristellaria, to illustrate the same principle
514, 5
261.
Another diagram of a logarithmic spiral
517
262.
A diagram of the logarithmic spiral of
Nautilus(Moseley)
519
263, 4.
Opercula of
Turboand of
Nerita(Moseley)
521, 2
265.
A section of the shell of
Melo ethiopicus525
266.
Shells of
Harpaand
Dolium, to illustrate generating curves and gene
526
267.
D’Orbigny’s Helicometer
529
268.
Section of a nautiloid shell, to shew the “protoconch”
531
269–73.
Diagrams of logarithmic spirals, of various angles
532–5
274, 6, 7.
Constructions for determining the angle of a logarithmic spiral
537, 8
275.
An ammonite, to shew its corrugated surface pattern
537
278–80.
Illustrations of the “angle of retardation”
542–4
281.
A shell of
Macroscaphites, to shew change of curvature
550
282.
Construction for determining the length of the coiled spire
551
283.
Section of the shell of
Triton corrugatus(Woodward)
554
284.
Lamellaria perspicuaand
Sigaretus haliotoides(
do.)
555
285, 6.
Sections of the shells of
Terebra maculataand
Trochus niloticus559, 60
287–9.
Diagrams illustrating the lines of growth on a lamellibranch shell
563–5
290.
Caprinella adversa(Woodward)
567
291.
Section of the shell of
Productus(Woods)
567
292.
The “skeletal loop” of
Terebratula(
do.)
568
293, 4.
The spiral arms of
Spiriferand of
Atrypa(
do.)
569
295–7.
Shells of
Cleodora,
Hyalaeaand other pteropods (Boas)
570, 1
298, 9.
Coordinate diagrams of the shell-outline in certain pteropods
572, 3
300.
Development of the shell of
Hyalaea tridentata(Tesch)
573
301.
Pteropod shells, of
Cleodoraand
Hyalaea, viewed from the side (Boas)
575
302, 3.
Diagrams of septa in a conical shell
579
304.
A section of
Nautilus, shewing the logarithmic spirals of the septa to which the shell-spiral is the evolute
581
305.
Cast of the interior of the shell of
Nautilus, to shew the contours of the septa at their junction with the shell-wall
582
306.
Ammonites Sowerbyi, to shew septal outlines (Zittel, after Steinmann and Döderlein)
584
307.
Suture-line of
Pinacoceras(Zittel, after Hauer)
584
308.
Shells of
Hastigerina, to shew the “mouth” (Brady)
588
309.
Nummulina antiquior(V. von Möller)
591
310.
Cornuspira foliaceaand
Operculina complanata(Brady)
594
311.
Miliolina pulchellaand
linnaeana(Brady)
596
312, 3.
Cyclammina cancellata(
do.), and diagrammatic figure of the same
596, 7
314.
Orbulina universa(Brady)
598
315.
Cristellaria reniformis(
do.)
600
316.
Discorbina bertheloti(
do.)
603
317.
Textularia trochusand
concava(
do.)
604
318.
Diagrammatic figure of a ram’s horns (Sir V. Brooke)
615
319.
Head of an Arabian wild goat (Sclater)
616
320.
Head of
Ovis Ammon, shewing St Venant’s curves
621
321.
St Venant’s diagram of a triangular prism under torsion (Thomson and Tait)
623
322.
Diagram of the same phenomenon in a ram’s horn
623
323.
Antlers of a Swedish elk (Lönnberg)
629
324.
Head and antlers of
Cervus duvauceli(Lydekker)
630
325, 6.
Diagrams of spiral phyllotaxis (P. G. Tait)
644, 5
327.
Further diagrams of phyllotaxis, to shew how various spiral appearances may arise out of one and the same angular leaf-divergence
648
328.
Diagrammatic outlines of various sea-urchins
664
329, 30.
Diagrams of the angle of branching in blood-vessels (Hess)
667, 8
331, 2.
Diagrams illustrating the flexure of a beam
674, 8
Fig. 1. Motor ganglion-cells, from the cervical spinal cord.
(From Minot, after Irving Hardesty.)
Fig. 2. Relative magnitudes of: A, human blood-corpuscle (7·5 µ in diameter); B, Bacillus anthracis (4 – 15 µ × 1 µ); C, various Micrococci (diam. 0·5 – 1 µ, rarely 2 µ); D, Micromonas progrediens, Schröter (diam. 0·15 µ).
Fig. 3. Curve of Growth in Man, from birth to 20 yrs ();) from Quetelet’s Belgian data. The upper curve of stature from Bowditch’s Boston data.
Fig. 4. Mean annual increments of stature (), Belgian and American.
Fig. 6. Percentage ratio, throughout life, of female weight to male; from Quetelet’s data.
Fig. 7. Curve of growth (in length or stature) of child, before and after birth. (From His and Rüssow’s data.)
Fig. 10. Curve of growth of bamboo (from Ostwald, after Kraus).
Fig. 11. Coefficients of variability of stature in Man (). from Boas and Wissler’s data.
Fig. 12. Growth in weight of Mouse. (After W. Ostwald.)
Fig. 13. Growth in weight of Silkworm. (From Ostwald, after Luciani and Lo Monaco.)
Fig. 14. Growth in weight of Tadpole. (From Ostwald, after Schaper.)
Fig. 15. Development of Eel; from Leptocephalus larvae to young Elver. (From Ostwald after Joh. Schmidt.)
Fig. 16. Growth in length of Spirogyra. (From Ostwald, after Hofmeister.)
Fig. 17. Pulsations of growth in Crocus, in micro-millimetres. (After Bose.)
Fig. 18. Relative growth in weight (in Man) of Brain, Heart, and whole Body.
Fig. 19. Ratio of stature in Man, to span of outstretched arms.
(From Quetelet’s data.)
Fig. 20. Rate of growth in successive zones near the tip of the bean-root.
Fig. 21. Changes in the weight-length ratio of Plaice, with increasing size.
Fig. 23. Variability of length of tail-forceps in a sample of Earwigs. (After Bateson, P. Z. S. 1892, p. 588.)
Fig. 24. Variability of length of body in a sample of Plaice.
Fig. 25. Relation of rate of growth to temperature in certain plants. (From Sachs’s data.)
Fig. 26. Relation of rate of growth to temperature in Maize. Observed values (after Köppen), and calculated curve.
Fig. 27. Relation of rate of growth to temperature in rootlets of Pea. (From Miss I. Leitch’s data.)
Fig. 28. Diagram shewing time taken (in days), at various temperatures (°C.), to reach certain stages of development in the Frog: viz. I, gastrula; II, medullary plate; III, closure of medullary folds; IV, tail-bud; V, tail and gills; VI, tail-fin; VII, operculum beginning; VIII, do. closing; IX, first appearance of hind-legs. (From Jenkinson, after O. Hertwig, 1898.)
Fig. 30. Half-yearly increments of growth, in cadets of various ages. (From Daffner’s data.)
Fig. 31. Periodic annual fluctuation in rate of growth of trees (in the southern hemisphere).
Fig. 32. Long-period fluctuation in rate of growth of Arizona trees (smoothed in 100-year periods), from A.D. 1390–1490 to A.D. 1810–1910.
Fig. 33. Brine-shrimps (Artemia), from more or less saline water. Upper figures shew tail-segment and tail-fins; lower figures, relative length of cephalothorax and abdomen. (After Abonyi.)
Fig. 35. Curve of regenerative growth in tadpoles’ tails. (From M. L. Durbin’s data.)
Fig. 40. Relation between the percentage amount of tail removed, the percentage restored, and the time required for its restoration. (From M. M. Ellis’s data.)
Fig. 41. Caryokinetic figure in a dividing cell (or blastomere) of the Trout’s egg. (After Prenant, from a preparation by Prof. P. Bouin.)
Fig. 42.
Fig. 43.
Fig. 52. Chromosomes, undergoing splitting and separation.
(After Hatschek and Flemming, diagrammatised.)
Fig. 53. Annular chromosomes, formed in the spermatogenesis of the Mole-cricket. (From Wilson, after Vom Rath.)
Fig. 54.
Fig. 57. Artificial caryokinesis (after Leduc), for comparison with Fig. 41, p. 169.
Fig. 58. Final stage in the first segmentation of the egg of Cerebratulus. (From Prenant, after Coe.)245
Fig. 59. Diagram of field of force with two similar poles.
Fig. 58. Final stage in the first segmentation of the egg of Cerebratulus. (From Prenant, after Coe.)245
Fig. 59. Diagram of field of force with two similar poles.
Fig. 60.
Fig. 61.
Fig. 62.
Fig. 63.
Fig. 66.
Fig. 67.
Fig. 68.
Fig. 69. Hair of Trianea, in glycerine. (After Berthold.)
Fig. 70. Phases of a Splash. (From Worthington.)
Fig. 71. A breaking wave. (From Worthington.)
Fig. 72. Calycles of Campanularian zoophytes. (A) C. integra; (B) C. groenlandica; (C) C. bispinosa; (D) C. raridentata.
Fig. 73. A flagellate “monad,”
Distigma proteus, Ehr. (After Saville Kent.)
Fig. 74.
Noctiluca miliaris.Fig. 73. A flagellate “monad,”
Distigma proteus, Ehr. (After Saville Kent.)
Fig. 74.
Noctiluca miliaris.Fig. 75. Various species of Vorticella. (Mostly after Saville Kent.)
Fig. 76. Various species of Salpingoeca.
Fig. 77. Various species of Tintinnus, Dinobryon and Codonella.
(After Saville Kent and others.)
Fig. 78. Vaginicola.
Fig. 79. Folliculina.
Fig. 80. Trachelophyllum. (After Wreszniowski.)
Fig. 81.
Fig. 82. Dinenympha gracilis, Leidy.
Fig. 83.
Fig. 84. Various species of Lagena. (After Brady.)
Fig. 85. (After Darling.)
Fig. 86.
Fig. 87.
Nodosaria scalaris, Batsch.
Fig. 88. Gonangia of Campanularians. (
a)
C. gracilis; (
b)
C. grandis. (After Allman.)
Fig. 87.
Nodosaria scalaris, Batsch.
Fig. 88. Gonangia of Campanularians. (
a)
C. gracilis; (
b)
C. grandis. (After Allman.)
Fig. 89. Various Foraminifera (after Brady), a, Nodosaria simplex; b, N. pygmaea; c, N. costulata; e, N. hispida; f, N. elata; d, Rheophax (Lituola) distans; g, Sagrina virgata.
Fig. 90. A, Trypanosoma tineae (after Minchin); B, Spirochaeta anodontae (after Fantham).
Fig. 91. A, Trichomonas muris, Hartmann; B, Trichomastix serpentis, Dobell; C, Trichomonas angusta, Alexeieff. (After Kofoid.)
Fig. 92. Herpetomonas assuming the undulatory membrane of a Trypanosome. (After D. L. Mackinnon.)
Fig. 93.
Fig. 94. Sperm-cells of Decapod Crustacea (after Koltzoff). a, Inachus scorpio; b, Galathea squamifera; c, do. after maceration, to shew spiral fibrillae.
Fig. 95. Sperm-cells of Inachus, as they appear in saline solutions of varying density. (After Koltzoff.)
Fig. 96. Sperm-cell of Dromia. (After Koltzoff.)
Fig. 97. A, B, Chondriosomes in kidney-cells, prior to and during secretory activity (after Barratt); C, do. in pancreas of frog (after Mathews).
Fig. 98. Adsorptive concentration of potassium salts in (1) cell of Pleurocarpus about to conjugate; (2) conjugating cells of Mesocarpus; (3) sprouting spores of Equisetum. (After Macallum.)
Fig. 99.
Fig. 102. (After Berthold.)
Fig. 103. Parenchyma of Maize.
Fig. 102. (After Berthold.)
Fig. 103. Parenchyma of Maize.
Fig. 104.
Fig. 105.
Fig. 106.
Fig. 107. Filaments, or chains of cells, in various lower Algae. (A) Nostoc; (B) Anabaena; (C) Rivularia; (D) Oscillatoria.
Fig. 108.
Fig. 109. Spore of Pellia. (After Campbell.)
Fig. 110. Cells of
Dictyota. (After Reinke.)
Fig. 111. Terminal and other cells of
Chara.
Fig. 110. Cells of
Dictyota. (After Reinke.)
Fig. 111. Terminal and other cells of
Chara.
Fig. 113.
Fig. 114.
Fig. 116.
Fig. 117. Various ways in which the four cells are co-arranged in the four-celled stage of the frog’s egg. (After Rauber.)
Fig. 118.
Fig. 119.
Fig. 120.
Fig. 121. Diagram of hexagonal cells. (After Bonanni.)
Fig. 122. An “artificial tissue,” formed by coloured drops of sodium chloride solution diffusing in a less dense solution of the same salt. (After Leduc.)
Fig. 124. Epidermis of Girardia. (After Goebel.)
Fig. 125. Soap-froth under pressure. (After Rhumbler.)
Fig. 126. From leaf of Elodea canadensis. (After Berthold.)
Fig. 127.
Lithostrotion Martini.(After Nicholson.)
Fig. 128.
Cyathophyllum hexagonum.(From Nicholson, after Zittel.)
Fig. 127.
Lithostrotion Martini.(After Nicholson.)
Fig. 128.
Cyathophyllum hexagonum.(From Nicholson, after Zittel.)
Fig. 129.
Arachnophyllum pentagonum.(After Nicholson.)
Fig. 130.
Heliolites.(After Woods.)
Fig. 129.
Arachnophyllum pentagonum.(After Nicholson.)
Fig. 130.
Heliolites.(After Woods.)
Fig. 131. Surface-views of Corals with undeveloped thecae and confluent septa. A, Thamnastraea; B, Comoseris. (From Nicholson, after Zittel.)
Fig. 132.
Fig. 133. Diagram of development of “stellate cells,” in pith of Juncus. (The dark, or shaded, areas represent the cells; the light areas being the gradually enlarging “intercellular spaces.”)
Fig. 134.
Fig. 135. Aggregations of four soap-bubbles, to shew various arrangements of the intermediate partition and polar furrows. (After Robert.)
Fig. 136. (After Berthold.)
Fig. 139.
Fig. 140.
Fig. 141.
Fig. 142. -shaped partitions: A, from Taonia atomaria (after Reinke); B, from paraphyses of Fucus; C, from rhizoids of Moss; D, from paraphyses of Polytrichum.
Fig. 143. Diagrammatic explanation of -shaped partition.
Fig. 144. Development of Erythrotrichia. (After Berthold.)
Fig. 145.
Fig. 146.
Fig. 147.
Fig. 148. Segmentation of frog’s egg, under artificial compression. (After Roux.)
Fig. 149.
Fig. 150.
Fig. 151.
Fig. 152.
Fig. 153. Diagram of flattened or discoid cell dividing into octants: to shew gradual tendency towards a position of equilibrium.
Fig. 154.
Fig. 155. Theoretical arrangement of successive partitions in a discoid cell; for comparison with Fig. 144.
Fig. 157. Sections of embryo of a moss. (After Kienitz-Gerloff.)
Fig. 158. Various possible arrangements of intermediate partitions, in groups of 4, 5, 6, 7 or 8 cells.
Fig. 159.
Fig. 160. Segmenting egg of
Trochus. (After Robert.)
Fig. 161. Two views of segmenting egg of
Cynthia partita. (After Conklin.)
Fig. 162. (a) Section of apical cone of Salvinia. (After Pringsheim394.) (b) Diagram of probable actual arrangement.
Fig. 163. Egg of
Pyrosoma. (After Korotneff).
Fig. 164. Egg of
Echinus, segmenting under pressure. (After Driesch.)
Fig. 165. (a) Part of segmenting egg of Cephalopod (after Watase); (b) probable actual arrangement.
Fig. 166. (a) Egg of Echinus; (b) do. of Nereis, under pressure. (After Driesch).
Fig. 168. Various modes of grouping of eight cells, at the dorsal or epiblastic pole of the frog’s egg. (After Rauber.)
Fig. 169.
Fig. 170. Aggregations of oil-drops. (After Roux.) Figs. 4–6 represent successive changes in a single system.
Fig. 171. (A) Asterolampra marylandica, Ehr.; (B, C) A. variabilis, Grev. (After Greville.)
Fig. 172. Section of Alcyonarian polype.
Fig. 173. Heterophyllia angulata. (After Nicholson.)
Fig. 175. Diagrammatic section of a Ctenophore (Eucharis).
Fig. 176. Diagrammatic arrangement of partitions, represented by skeletal rods, in larval Echinoderm (Ophiura).
Fig. 178. Diagrammatic development of Stomata in Sedum. (Cf. fig. in Sachs’s Botany, 1882, p. 103.)
Fig. 180. Various pollen-grains and spores (after Berthold, Campbell, Goebel and others). (1) Epilobium; (2) Passiflora; (3) Neottia; (4) Periploca graeca; (5) Apocynum; (6) Erica; (7) Spore of Osmunda; (8) Tetraspore of Callithamnion.
Fig. 181. Dividing spore of Anthoceros. (After Campbell.)
Fig. 182.
Fig. 183. Development of Sphagnum. (After Campbell.)
Fig. 185. Gemma of Moss. (After Campbell.)
Fig. 186. Development of antheridium of Riccia. (After Campbell.)
Fig. 187. Section of growing shoot of Selaginella, diagrammatic.
Fig. 188. Embryo of Jungermannia. (After Kienitz-Gerloff.)
Fig. 187. Section of growing shoot of Selaginella, diagrammatic.
Fig. 188. Embryo of Jungermannia. (After Kienitz-Gerloff.)
Fig. 190. Development of sporangium of Osmunda. (After Bower.)
Fig. 191. (A, B,) Sections of younger and older embryos of Phascum; (C) do. of Adiantum. (After Kienitz-Gerloff.)
Fig. 192. Section through frond of Girardia sphacelaria. (After Goebel.)
Fig. 193. Development of antheridium of Pteris. (After Strasbürger.)
Fig. 194. Alcyonarian spicules: Siphonogorgia and Anthogorgia. (After Studer.)
Fig. 195. Calcospherites, or concretions of calcium carbonate, deposited in white of egg. (After Harting.)
Fig. 196. A single calcospherite, with central “nucleus,” and striated, iridescent border. (After Harting.)
Fig. 198, A. Section of shell of Mya; B. Section of hinge-tooth of do. (After Carpenter.)
Fig. 199. Large irregular calcareous concretions, or spicules, deposited in a piece of dead cartilage, in presence of calcium phosphate. (After Harting.)
Fig. 200. Additional illustrations of Alcyonarian spicules: Eunicea. (After Studer.)
Fig. 201. A “crust” of close-packed calcareous concretions, precipitated at the surface of an albuminous solution. (After Harting.)
Fig. 202. Aggregated calcospherites. (After Harting.)
Fig. 204. Conostats. (After Harting.)
Fig. 205. Liesegang’s Rings. (After Leduc.)
Fig. 206. Relay-crystals of common salt. (After Bowman.)
Fig. 207. Wheel-like crystals in a colloid. (After Bowman.)
Fig. 208.
Fig. 209. Otoliths of Plaice, showing four zones or “age-rings.” (After Wallace.)
Fig. 210. Close-packed calcospherites, or so-called “spicules,” of Astrosclera. (After Lister.)
Fig. 211. Sponge and Holothurian spicules.
Fig. 213. An “amphidisc” of Hyalonema.
Fig. 214. Spicules of Grantia and other calcareous sponges. (After Haeckel.)
Fig. 218.
Fig. 219. Arenaceous Foraminifera; Astrorhiza limicola and arenaria. (From Brady’s Challenger Monograph.)
Fig. 220. “Reticulum plasmatique.” (After Carnoy.)
Fig. 221. Aulonia hexagona, Hkl.
Fig. 222. Actinomma arcadophorum, Hkl.
Fig. 223.
Ethmosphaera conosiphonia, Hkl.
Fig. 224. Portions of shells of two “species” of
Cenosphaera: upper figure,
C. favosa, lower,
C. vesparia, Hkl.
Fig. 223.
Ethmosphaera conosiphonia, Hkl.
Fig. 224. Portions of shells of two “species” of
Cenosphaera: upper figure,
C. favosa, lower,
C. vesparia, Hkl.
Fig. 225. Aulastrum triceros, Hkl.
Fig. 226.
Fig. 227. A Nassellarian skeleton,
Callimitra carolotae, Hkl.
Fig. 226.
Fig. 227. A Nassellarian skeleton,
Callimitra carolotae, Hkl.
Fig. 228. An isolated portion of the skeleton of Dictyocha.
Fig. 230.
Fig. 231. Skeletons of various Radiolarians, after Haeckel. 1. Circoporus sexfurcus; 2. C. octahedrus; 3. Circogonia icosahedra; 4. Circospathis novena; 5. Circorrhegma dodecahedra.
Fig. 232. Dorataspis sp.; diagrammatic.
Fig. 233. Phatnaspis cristata, Hkl.
Fig. 235. Phractaspis prototypus, Hkl.
Fig. 236. Annular and spiral thickenings in the walls of plant-cells.
Fig. 237. The shell of Nautilus pompilius, from a radiograph: to shew the logarithmic spiral of the shell, together with the arrangement of the internal septa. (From Messrs Green and Gardiner, in Proc. Malacol. Soc. II, 1897.)
Fig. 238. A Foraminiferal shell (Globigerina).
Fig. 239.
Fig. 243. A, a helicoid, B, a scorpioid cyme.
Fig. 244.
Fig. 245.
Fig. 248.
Fig. 258. A shell of Haliotis, with two of the many lines of growth, or generating curves, marked out in black: the areas bounded by these lines of growth being in all cases “gnomons” to the pre-existing shell.
Fig. 259. A spiral foraminifer (Pulvinulina), to show how each successive chamber continues the symmetry of, or constitutes a gnomon to, the rest of the structure.
Fig. 261.
Fig. 262.
Fig. 263. Operculum of Turbo.
Fig. 265. Melo ethiopicus, L.
Fig. 266. 1, Harpa; 2, Dolium. The ridges on the shell correspond in (1) to generating curves, in (2) to generating spirals.
Fig. 267. D’Orbigny’s Helicometer.
Fig. 268.
Fig. 269.
Fig. 274.
Fig. 275. An Ammonite, to shew corrugated surface-pattern.
Fig. 276.
Fig. 278.
Fig. 281. An ammonitoid shell (Macroscaphites) to shew change of curvature.
Fig. 282.
Fig. 283. Section of a spiral, or turbinate, univalve, Triton corrugatus, Lam. (From Woodward.)
Fig. 284. A, Lamellaria perspicua; B, Sigaretus haliotoides.
(After Woodward.)
Fig. 285. Terebra maculata, L.
Fig. 287.
Fig. 290.
Caprinella adversa.(After Woodward.)
Fig. 291. Section of
Productus(
Strophomena) sp. (From Woods.)
Fig. 290.
Caprinella adversa.(After Woodward.)
Fig. 291. Section of
Productus(
Strophomena) sp. (From Woods.)
Fig. 292. Skeletal loop of Terebratula. (From Woods.)
Fig. 293. Spiral arms of
Spirifer. (From Woods.)
Fig. 294. Inwardly directed spiral arms of
Atrypa.
Fig. 295. Pteropod shells: (1) Cuvierina columnella; (2) Cleodora chierchiae; (3) C. pygmaea. (After Boas.)
Fig. 298. Cleodora cuspidata.
Fig. 300. Development of the shell of Hyalaea (Cavolinia) tridentata, Forskal: the earlier stages being the “Pleuropus longifilis” of Troschel. (After Tesch.)
Fig. 301. Pteropod shells, from the side: (1) Cleodora cuspidata; (2) Hyalaea longirostris; (3) H. trispinosa. (After Boas.)
Fig. 302.
Fig. 304. Section of Nautilus, shewing the contour of the septa in the median plane: the septa being (in this plane) logarithmic spirals, of which the shell-spiral is the evolute.
Fig. 305. Cast of the interior of Nautilus: to shew the contours of the septa at their junction with the shell-wall.
Fig. 306. Ammonites (Sonninia) Sowerbyi. (From Zittel, after Steinmann and Döderlein.)
Fig. 307. Suture-line of a Triassic Ammonite (Pinacoceras). (From Zittel, after Hauer.)
Fig. 308. Hastigerina sp.; to shew the “mouth.”
Fig. 309. Nummulina antiquior, R. and V. (After V. von Möller.)
Fig. 310. A, Cornuspira foliacea, Phil.; B, Operculina complanata, Defr.
Fig. 311. 1, 2, Miliolina pulchella, d’Orb.; 3–5, M. linnaeana, d’Orb. (After Brady.)
Fig. 312. Cyclammina cancellata, Brady.
Fig. 314. Orbulina universa, d’Orb.
Fig. 315. Cristellaria reniformis, d’Orb.
Fig. 316. Discorbina bertheloti, d’Orb.
Fig. 317. A, Tertularia trochus, d’Orb. B, T. concava, Karrer.
Fig. 318. Diagram of Ram’s horns. (After Sir Vincent Brooke, from P.Z.S.) a, frontal; b, orbital; c, nuchal surface.
Fig. 319. Head of Arabian Wild Goat, Capra sinaitica. (After Sclater, from P.Z.S.)
Fig. 320. Head of Ovis Ammon, shewing St Venant’s curves.
Fig. 321.
Fig. 322.
Fig. 321.
Fig. 322.
Fig. 323. Antlers of Swedish Elk. (After Lönnberg, from P.Z.S.)
Fig. 324. Head and antlers of a Stag (Cervus Duvauceli). (After Lydekker, from P.Z.S.)
Fig. 325.
Fig. 327.
Fig. 328. Diagrammatic vertical outlines of various Sea-urchins: A, Palaeechinus; B, Echinus acutus; C, Cidaris; D, D′ Coelopleurus; E, E′ Genicopatagus; F, Phormosoma luculenter; G, P. tenuis; H, Asthenosoma; I, Urechinus.
Fig. 329.
Fig. 331.
.
An example of the mode of arrangement of bast-fibres in a plant-stem (Schwendener)
680
334.
Section of the head of a femur, to shew its trabecular structure (Schäfer, after Robinson)
681
335.
Comparative diagrams of a crane-head and the head of a femur (Culmann and H. Meyer)
682
336.
Diagram of stress-lines in the human foot (Sir D. MacAlister, after H. Meyer)
684
337.
Trabecular structure of the
os calcis(
do.)
685
338.
Diagram of shearing-stress in a loaded pillar
686
339.
Diagrams of tied arch, and bowstring girder (Fidler)
693
340, 1.
Diagrams of a bridge: shewing proposed span, the corresponding stress-diagram and reciprocal plan of construction (
do.)
696
342.
A loaded bracket and its reciprocal construction-diagram (Culmann)
697
343, 4.
A cantilever bridge, with its reciprocal diagrams (Fidler)
698
345.
A two-armed cantilever of the Forth Bridge (
do.)
700
346.
A two-armed cantilever with load distributed over two pier-heads, as in the quadrupedal skeleton
700
347–9.
Stress-diagrams. or diagrams of bending moments, in the backbones of the horse, of a Dinosaur, and of
Titanotherium701–4
350.
The skeleton of
Stegosaurus707
351.
Bending-moments in a beam with fixed ends, to illustrate the mechanics of chevron-bones
709
352, 3.
Coordinate diagrams of a circle, and its deformation into an ellipse
729
354.
Comparison, by means of Cartesian coordinates, of the cannon-bones of various ruminant animals
729
355, 6.
Logarithmic coordinates, and the circle of Fig. 352 inscribed therein
729, 31
357, 8.
Diagrams of oblique and radial coordinates
731
359.
Lanceolate, ovate and cordate leaves, compared by the help of radial coordinates
732
360.
A leaf of
Begonia daedalea733
361.
A network of logarithmic spiral coordinates
735
362, 3.
Feet of ox, sheep and giraffe, compared by means of Cartesian coordinates
738, 40
364, 6.
“Proportional diagrams” of human physiognomy (Albert Dürer)
740, 2
365.
Median and lateral toes of a tapir, compared by means of rectangular and oblique coordinates
741
367, 8.
A comparison of the copepods
Oithonaand
Sapphirina742
369.
The carapaces of certain crabs,
Geryon,
Corystesand others, compared by means of rectilinear and curvilinear coordinates
744
370.
A comparison of certain amphipods,
Harpinia,
Stegocephalusand
Hyperia746
371.
The calycles of certain campanularian zoophytes, inscribed in corresponding Cartesian networks
747
372.
The calycles of certain species of
Aglaophenia, similarly compared by means of curvilinear coordinates
748
373, 4.
The fishes
Argyropelecusand
Sternoptyx, compared by means of rectangular and oblique coordinate systems
748
375, 6.
Scarusand
Pomacanthus, similarly compared by means of rectangular and coaxial systems
749
377–80.
A comparison of the fishes
Polyprion,
Pseudopriacanthus,
Scorpaenaand
Antigonia750
381, 2.
A similar comparison of
Diodonand
Orthagoriscus751
383.
The same of various crocodiles:
C. porosus,
C. americanusand
Notosuchus terrestris753
384.
The pelvic girdles of
Stegosaurusand
Camptosaurus754
385, 6.
The shoulder-girdles of
Cryptocleidusand of
Ichthyosaurus755
387.
The skulls of
Dimorphodonand of
Pteranodon756
388–92.
The pelves of
Archaeopteryxand of
Apatorniscompared, and a method illustrated whereby intermediate configurations may be found by interpolation (G. Heilmann)
757–9
393.
The same pelves, together with three of the intermediate or interpolated forms
760
394, 5.
Comparison of the skulls of two extinct rhinoceroses,
Hyrachyusand
Aceratherium(Osborn)
761
396.
Occipital views of various extinct rhinoceroses (
do.)
762
397–400.
Comparison with each other, and with the skull of
Hyrachyus, of the skulls of
Titanotherium, tapir, horse and rabbit
763, 4
401, 2.
Coordinate diagrams of the skulls of
Eohippusand of
Equus, with various actual and hypothetical intermediate types (Heilmann)
765–7
403.
A comparison of various human scapulae (Dwight)
769
404.
A human skull, inscribed in Cartesian coordinates
770
405.
The same coordinates on a new projection, adapted to the skull of the chimpanzee
770
406.
Chimpanzee’s skull, inscribed in the network of Fig. 405
771
407, 8.
Corresponding diagrams of a baboon’s skull, and of a dog’s
771, 3
Fig. 333.
Fig. 334. Head of the human femur in section. (After Schäfer, from a photo by Prof. A. Robinson.)
Fig. 335. Crane-head and femur. (After Culmann and H. Meyer.)
Fig. 336. Diagram of stress-lines in the human foot. (From Sir D. MacAlister, after H. Meyer.)
Fig. 337. Trabecular structure of the os calcis. (From MacAlister.)
Fig. 338.
Fig. 339.
Fig. 340. A, Span of proposed bridge. B, Stress diagram, or diagram of bending-moments628.
Fig. 342.
Fig. 343.
Fig. 345. A two-armed cantilever of the Forth Bridge. Thick lines, compression-members (bones); thin lines, tension-members (ligaments).
Fig. 346.
Fig. 347. Stress-diagram of horse’s backbone.
Fig. 350. Diagram of Stegosaurus.
Fig. 351.
Fig. 352.
Fig. 353.
Fig. 354.
Fig. 355.
Fig. 354.
Fig. 355.
Fig. 357.
Fig. 359.
Fig. 360. Begonia daedalea.
Fig. 361.
Fig. 362.
Fig. 364. (After Albert Dürer.)
Fig. 365.
Fig. 367.
Oithona nana.Fig. 368.
Sapphirina.Fig. 369. Carapaces of various crabs. 1, Geryon; 2, Corystes; 3, Scyramathia; 4, Paralomis; 5, Lupa; 6, Chorinus.
Fig 370. 1. Harpinia plumosa Kr. 2. Stegocephalus inflatus Kr. 3. Hyperia galba.
Fig. 371. a, Campanularia macroscyphus, Allm.; b, Gonothyraea hyalina, Hincks; c, Clytia Johnstoni, Alder.
Fig. 372. a, Cladocarpus crenatus, F.; b, Aglaophenia pluma, L.; c, A. rhynchocarpa, A.; d, A cornuta, K.; e, A. ramulosa, K.
Fig. 373.
Argyropelecus Olfersi.Fig. 374.
Sternoptyx diaphana.Fig. 375.
Scarussp.
Fig. 376.
Pomacanthus.Fig. 377.
Polyprion.Fig. 378.
Pseudopriacanthus altus.Fig. 381.
Diodon.Fig. 382.
Orthagoriscus.Fig. 383. A, Crocodilus porosus. B, C. americanus. C, Notosuchus terrestris.
Fig. 384. Pelvis of (A) Stegosaurus; (B) Camptosaurus.
Fig. 385. Shoulder-girdle of Cryptocleidus. a, young; b, adult.
Fig. 387. a, Skull of Dimorphodon. b, Skull of Pteranodon.
Fig. 388. Pelvis of Archaeopteryx.
Fig. 393. The pelves of Archaeopteryx and of Apatornis, with three transitional types interpolated between them.
Fig. 394. Skull of Hyrachyus agrarius. (After Osborn.)
Fig. 396. Occipital view of the skulls of various extinct rhinoceroses (Aceratherium spp.). (After Osborn.)
Fig. 397.
Titanotherium robustum.
Fig. 398. Tapir’s skull.
Fig. 401. A, outline diagram of the Cartesian co-ordinates of the skull of Hyracotherium or Eohippus, as shewn in Fig. 402, A. H, outline of the corresponding projection of the horse’s skull. B–G, intermediate, or interpolated, outlines.
Fig. 403. Human scapulae (after Dwight). A, Caucasian; B, Negro; C, North American Indian (from Kentucky Mountains).
Fig. 404. Human skull.
Fig. 405. Co-ordinates of chimpanzee’s skull, as a projection of the Cartesian co-ordinates of Fig. 404.
Fig. 406. Skull of chimpanzee.
Fig. 407. Skull of baboon.
Fig. 406. Skull of chimpanzee.
Fig. 407. Skull of baboon.
“Cum formarum naturalium et corporalium esse non consistat nisi in unione ad materiam, ejusdem agentis esse videtur eas producere cujus est materiam transmutare. Secundo, quia cum hujusmodi formae non excedant virtutem et ordinem et facultatem principiorum agentium in natura, nulla videtur necessitas eorum originem in principia reducere altiora.” Aquinas, De Pot. Q. iii, a, 11. (Quoted in Brit. Assoc. Address, Section D, 1911.)
“...I would that all other natural phenomena might similarly be deduced from mechanical principles. For many things move me to suspect that everything depends upon certain forces, in virtue of which the particles of bodies, through forces not yet understood, are either impelled together so as to cohere in regular figures, or are repelled and recede from one another.” Newton, in Preface to the Principia. (Quoted by Mr W. Spottiswoode, Brit. Assoc. Presidential Address, 1878.)
“When Science shall have subjected all natural phenomena to the laws of Theoretical Mechanics, when she shall be able to predict the result of every combination as unerringly as Hamilton predicted conical refraction, or Adams revealed to us the existence of Neptune,—that we cannot say. That day may never come, and it is certainly far in the dim future. We may not anticipate it, we may not even call it possible. But none the less are we bound to look to that day, and to labour for it as the crowning triumph of Science:—when Theoretical Mechanics shall be recognised as the key to every physical enigma, the chart for every traveller through the dark Infinite of Nature.” J. H. Jellett, in Brit. Assoc. Address, Section A, 1874.
CHAPTER I INTRODUCTORY
Of the chemistry of his day and generation, Kant declared that it was “a science, but not science,”—“eine Wissenschaft, aber nicht Wissenschaft”; for that the criterion of physical science lay in its relation to mathematics. And a hundred years later Du Bois Reymond, profound student of the many sciences on which physiology is based, recalled and reiterated the old saying, declaring that chemistry would only reach the rank of science, in the high and strict sense, when it should be found possible to explain chemical reactions in the light of their causal relation to the velocities, tensions and conditions of equilibrium of the component molecules; that, in short, the chemistry of the future must deal with molecular mechanics, by the methods and in the strict language of mathematics, as the astronomy of Newton and Laplace dealt with the stars in their courses. We know how great a step has been made towards this distant and once hopeless goal, as Kant defined it, since van’t Hoff laid the firm foundations of a mathematical chemistry, and earned his proud epitaph, Physicam chemiae adiunxit1.
We need not wait for the full realisation of Kant’s desire, in order to apply to the natural sciences the principle which he urged. Though chemistry fall short of its ultimate goal in mathematical mechanics, nevertheless physiology is vastly strengthened and enlarged by making use of the chemistry, as of the physics, of the age. Little by little it draws nearer to our conception of a true science, with each branch of physical science which it {2} brings into relation with itself: with every physical law and every mathematical theorem which it learns to take into its employ. Between the physiology of Haller, fine as it was, and that of Helmholtz, Ludwig, Claude Bernard, there was all the difference in the world.
As soon as we adventure on the paths of the physicist, we learn to weigh and to measure, to deal with time and space and mass and their related concepts, and to find more and more our knowledge expressed and our needs satisfied through the concept of number, as in the dreams and visions of Plato and Pythagoras; for modern chemistry would have gladdened the hearts of those great philosophic dreamers.
But the zoologist or morphologist has been slow, where the physiologist has long been eager, to invoke the aid of the physical or mathematical sciences; and the reasons for this difference lie deep, and in part are rooted in old traditions. The zoologist has scarce begun to dream of defining, in mathematical language, even the simpler organic forms. When he finds a simple geometrical construction, for instance in the honey-comb, he would fain refer it to psychical instinct or design rather than to the operation of physical forces; when he sees in snail, or nautilus, or tiny foraminiferal or radiolarian shell, a close approach to the perfect sphere or spiral, he is prone, of old habit, to believe that it is after all something more than a spiral or a sphere, and that in this “something more” there lies what neither physics nor mathematics can explain. In short he is deeply reluctant to compare the living with the dead, or to explain by geometry or by dynamics the things which have their part in the mystery of life. Moreover he is little inclined to feel the need of such explanations or of such extension of his field of thought. He is not without some justification if he feels that in admiration of nature’s handiwork he has an horizon open before his eyes as wide as any man requires. He has the help of many fascinating theories within the bounds of his own science, which, though a little lacking in precision, serve the purpose of ordering his thoughts and of suggesting new objects of enquiry. His art of classification becomes a ceaseless and an endless search after the blood-relationships of things living, and the pedigrees of things {3} dead and gone. The facts of embryology become for him, as Wolff, von Baer and Fritz Müller proclaimed, a record not only of the life-history of the individual but of the annals of its race. The facts of geographical distribution or even of the migration of birds lead on and on to speculations regarding lost continents, sunken islands, or bridges across ancient seas. Every nesting bird, every ant-hill or spider’s web displays its psychological problems of instinct or intelligence. Above all, in things both great and small, the naturalist is rightfully impressed, and finally engrossed, by the peculiar beauty which is manifested in apparent fitness or “adaptation,”—the flower for the bee, the berry for the bird.
Time out of mind, it has been by way of the “final cause,” by the teleological concept of “end,” of “purpose,” or of “design,” in one or another of its many forms (for its moods are many), that men have been chiefly wont to explain the phenomena of the living world; and it will be so while men have eyes to see and ears to hear withal. With Galen, as with Aristotle, it was the physician’s way; with John Ray, as with Aristotle, it was the naturalist’s way; with Kant, as with Aristotle, it was the philosopher’s way. It was the old Hebrew way, and has its splendid setting in the story that God made “every plant of the field before it was in the earth, and every herb of the field before it grew.” It is a common way, and a great way; for it brings with it a glimpse of a great vision, and it lies deep as the love of nature in the hearts of men.
Half overshadowing the “efficient” or physical cause, the argument of the final cause appears in eighteenth century physics, in the hands of such men as Euler2 and Maupertuis, to whom Leibniz3 had passed it on. Half overshadowed by the mechanical concept, it runs through Claude Bernard’s Leçons sur les {4} phénomènes de la Vie4, and abides in much of modern physiology5. Inherited from Hegel, it dominated Oken’s Naturphilosophie and lingered among his later disciples, who were wont to liken the course of organic evolution not to the straggling branches of a tree, but to the building of a temple, divinely planned, and the crowning of it with its polished minarets6.
It is retained, somewhat crudely, in modern embryology, by those who see in the early processes of growth a significance “rather prospective than retrospective,” such that the embryonic phenomena must be “referred directly to their usefulness in building the body of the future animal7”:—which is no more, and no less, than to say, with Aristotle, that the organism is the τέλος, or final cause, of its own processes of generation and development. It is writ large in that Entelechy8 which Driesch rediscovered, and which he made known to many who had neither learned of it from Aristotle, nor studied it with Leibniz, nor laughed at it with Voltaire. And, though it is in a very curious way, we are told that teleology was “refounded, reformed or rehabilitated9” by Darwin’s theory of natural selection, whereby “every variety of form and colour was urgently and absolutely called upon to produce its title to existence either as an active useful agent, or as a survival” of such active usefulness in the past. But in this last, and very important case, we have reached a “teleology” without a τέλος, {5} as men like Butler and Janet have been prompt to shew: a teleology in which the final cause becomes little more, if anything, than the mere expression or resultant of a process of sifting out of the good from the bad, or of the better from the worse, in short of a process of mechanism10. The apparent manifestations of “purpose” or adaptation become part of a mechanical philosophy, according to which “chaque chose finit toujours par s’accommoder à son milieu11.” In short, by a road which resembles but is not the same as Maupertuis’s road, we find our way to the very world in which we are living, and find that if it be not, it is ever tending to become, “the best of all possible worlds12.”
But the use of the teleological principle is but one way, not the whole or the only way, by which we may seek to learn how things came to be, and to take their places in the harmonious complexity of the world. To seek not for ends but for “antecedents” is the way of the physicist, who finds “causes” in what he has learned to recognise as fundamental properties, or inseparable concomitants, or unchanging laws, of matter and of energy. In Aristotle’s parable, the house is there that men may live in it; but it is also there because the builders have laid one stone upon another: and it is as a mechanism, or a mechanical construction, that the physicist looks upon the world. Like warp and woof, mechanism and teleology are interwoven together, and we must not cleave to the one and despise the other; for their union is “rooted in the very nature of totality13.”
Nevertheless, when philosophy bids us hearken and obey the lessons both of mechanical and of teleological interpretation, the precept is hard to follow: so that oftentimes it has come to pass, just as in Bacon’s day, that a leaning to the side of the final cause “hath intercepted the severe and diligent inquiry of all {6} real and physical causes,” and has brought it about that “the search of the physical cause hath been neglected and passed in silence.” So long and so far as “fortuitous variation14” and the “survival of the fittest” remain engrained as fundamental and satisfactory hypotheses in the philosophy of biology, so long will these “satisfactory and specious causes” tend to stay “severe and diligent inquiry,” “to the great arrest and prejudice of future discovery.”
The difficulties which surround the concept of active or “real” causation, in Bacon’s sense of the word, difficulties of which Hume and Locke and Aristotle were little aware, need scarcely hinder us in our physical enquiry. As students of mathematical and of empirical physics, we are content to deal with those antecedents, or concomitants, of our phenomena, without which the phenomenon does not occur,—with causes, in short, which, aliae ex aliis aptae et necessitate nexae, are no more, and no less, than conditions sine quâ non. Our purpose is still adequately fulfilled: inasmuch as we are still enabled to correlate, and to equate, our particular phenomena with more and ever more of the physical phenomena around, and so to weave a web of connection and interdependence which shall serve our turn, though the metaphysician withhold from that interdependence the title of causality. We come in touch with what the schoolmen called a ratio cognoscendi, though the true ratio efficiendi is still enwrapped in many mysteries. And so handled, the quest of physical causes merges with another great Aristotelian theme,—the search for relations between things apparently disconnected, and for “similitude in things to common view unlike.” Newton did not shew the cause of the apple falling, but he shewed a similitude between the apple and the stars.
Moreover, the naturalist and the physicist will continue to speak of “causes,” just as of old, though it may be with some mental reservations: for, as a French philosopher said, in a kindred difficulty: “ce sont là des manières de s’exprimer, {7} et si elles sont interdites il faut renoncer à parler de ces choses.”
The search for differences or essential contrasts between the phenomena of organic and inorganic, of animate and inanimate things has occupied many mens’ minds, while the search for community of principles, or essential similitudes, has been followed by few; and the contrasts are apt to loom too large, great as they may be. M. Dunan, discussing the “Problème de la Vie15” in an essay which M. Bergson greatly commends, declares: “Les lois physico-chimiques sont aveugles et brutales; là où elles règnent seules, au lieu d’un ordre et d’un concert, il ne peut y avoir qu’incohérence et chaos.” But the physicist proclaims aloud that the physical phenomena which meet us by the way have their manifestations of form, not less beautiful and scarce less varied than those which move us to admiration among living things. The waves of the sea, the little ripples on the shore, the sweeping curve of the sandy bay between its headlands, the outline of the hills, the shape of the clouds, all these are so many riddles of form, so many problems of morphology, and all of them the physicist can more or less easily read and adequately solve: solving them by reference to their antecedent phenomena, in the material system of mechanical forces to which they belong, and to which we interpret them as being due. They have also, doubtless, their immanent teleological significance; but it is on another plane of thought from the physicist’s that we contemplate their intrinsic harmony and perfection, and “see that they are good.”
Nor is it otherwise with the material forms of living things. Cell and tissue, shell and bone, leaf and flower, are so many portions of matter, and it is in obedience to the laws of physics that their particles have been moved, moulded and conformed16. {8} They are no exception to the rule that Θεὸς ἀεὶ γεωμετρεῖ. Their problems of form are in the first instance mathematical problems, and their problems of growth are essentially physical problems; and the morphologist is, ipso facto, a student of physical science.
Apart from the physico-chemical problems of modern physiology, the road of physico-mathematical or dynamical investigation in morphology has had few to follow it; but the pathway is old. The way of the old Ionian physicians, of Anaxagoras17, of Empedocles and his disciples in the days before Aristotle, lay just by that highwayside. It was Galileo’s and Borelli’s way. It was little trodden for long afterwards, but once in a while Swammerdam and Réaumur looked that way. And of later years, Moseley and Meyer, Berthold, Errera and Roux have been among the little band of travellers. We need not wonder if the way be hard to follow, and if these wayfarers have yet gathered little. A harvest has been reaped by others, and the gleaning of the grapes is slow.
It behoves us always to remember that in physics it has taken great men to discover simple things. They are very great names indeed that we couple with the explanation of the path of a stone, the droop of a chain, the tints of a bubble, the shadows in a cup. It is but the slightest adumbration of a dynamical morphology that we can hope to have, until the physicist and the mathematician shall have made these problems of ours their own, or till a new Boscovich shall have written for the naturalist the new Theoria Philosophiae Naturalis.
How far, even then, mathematics will suffice to describe, and physics to explain, the fabric of the body no man can foresee. It may be that all the laws of energy, and all the properties of matter, and all the chemistry of all the colloids are as powerless to explain the body as they are impotent to comprehend the soul. For my part, I think it is not so. Of how it is that the soul informs the body, physical science teaches me nothing: consciousness is not explained to my comprehension by all the nerve-paths and “neurones” of the physiologist; nor do I ask of physics how goodness shines in one man’s face, and evil betrays itself in another. But of the construction and growth and working {9} of the body, as of all that is of the earth earthy, physical science is, in my humble opinion, our only teacher and guide18.
Often and often it happens that our physical knowledge is inadequate to explain the mechanical working of the organism; the phenomena are superlatively complex, the procedure is involved and entangled, and the investigation has occupied but a few short lives of men. When physical science falls short of explaining the order which reigns throughout these manifold phenomena,—an order more characteristic in its totality than any of its phenomena in themselves,—men hasten to invoke a guiding principle, an entelechy, or call it what you will. But all the while, so far as I am aware, no physical law, any more than that of gravity itself, not even among the puzzles of chemical “stereometry,” or of physiological “surface-action” or “osmosis,” is known to be transgressed by the bodily mechanism.
Some physicists declare, as Maxwell did, that atoms or molecules more complicated by far than the chemist’s hypotheses demand are requisite to explain the phenomena of life. If what is implied be an explanation of psychical phenomena, let the point be granted at once; we may go yet further, and decline, with Maxwell, to believe that anything of the nature of physical complexity, however exalted, could ever suffice. Other physicists, like Auerbach19, or Larmor20, or Joly21, assure us that our laws of thermodynamics do not suffice, or are “inappropriate,” to explain the maintenance or (in Joly’s phrase) the “accelerative absorption” {10} of the bodily energies, and the long battle against the cold and darkness which is death. With these weighty problems I am not for the moment concerned. My sole purpose is to correlate with mathematical statement and physical law certain of the simpler outward phenomena of organic growth and structure or form: while all the while regarding, ex hypothesi, for the purposes of this correlation, the fabric of the organism as a material and mechanical configuration.
Physical science and philosophy stand side by side, and one upholds the other. Without something of the strength of physics, philosophy would be weak; and without something of philosophy’s wealth, physical science would be poor. “Rien ne retirera du tissu de la science les fils d’or que la main du philosophe y a introduits22.” But there are fields where each, for a while at least, must work alone; and where physical science reaches its limitations, physical science itself must help us to discover. Meanwhile the appropriate and legitimate postulate of the physicist, in approaching the physical problems of the body, is that with these physical phenomena no alien influence interferes. But the postulate, though it is certainly legitimate, and though it is the proper and necessary prelude to scientific enquiry, may some day be proven to be untrue; and its disproof will not be to the physicist’s confusion, but will come as his reward. In dealing with forms which are so concomitant with life that they are seemingly controlled by life, it is in no spirit of arrogant assertiveness that the physicist begins his argument, after the fashion of a most illustrious exemplar, with the old formulary of scholastic challenge,—An Vita sit? Dico quod non.
The terms Form and Growth, which make up the title of this little book, are to be understood, as I need hardly say, in their relation to the science of organisms. We want to see how, in some cases at least, the forms of living things, and of the parts of living things, can be explained by physical considerations, and to realise that, in general, no organic forms exist save such as are in conformity with ordinary physical laws. And while growth is a somewhat vague word for a complex matter, which may {11} depend on various things, from simple imbibition of water to the complicated results of the chemistry of nutrition, it deserves to be studied in relation to form, whether it proceed by simple increase of size without obvious alteration of form, or whether it so proceed as to bring about a gradual change of form and the slow development of a more or less complicated structure.
In the Newtonian language of elementary physics, force is recognised by its action in producing or in changing motion, or in preventing change of motion or in maintaining rest. When we deal with matter in the concrete, force does not, strictly speaking, enter into the question, for force, unlike matter, has no independent objective existence. It is energy in its various forms, known or unknown, that acts upon matter. But when we abstract our thoughts from the material to its form, or from the thing moved to its motions, when we deal with the subjective conceptions of form, or movement, or the movements that change of form implies, then force is the appropriate term for our conception of the causes by which these forms and changes of form are brought about. When we use the term force, we use it, as the physicist always does, for the sake of brevity, using a symbol for the magnitude and direction of an action in reference to the symbol or diagram of a material thing. It is a term as subjective and symbolic as form itself, and so is appropriately to be used in connection therewith.
The form, then, of any portion of matter, whether it be living or dead, and the changes of form that are apparent in its movements and in its growth, may in all cases alike be described as due to the action of force. In short, the form of an object is a “diagram of forces,” in this sense, at least, that from it we can judge of or deduce the forces that are acting or have acted upon it: in this strict and particular sense, it is a diagram,—in the case of a solid, of the forces that have been impressed upon it when its conformation was produced, together with those that enable it to retain its conformation; in the case of a liquid (or of a gas) of the forces that are for the moment acting on it to restrain or balance its own inherent mobility. In an organism, great or small, it is not merely the nature of the motions of the living substance that we must interpret in terms of force (according to kinetics), but also {12} the conformation of the organism itself, whose permanence or equilibrium is explained by the interaction or balance of forces, as described in statics.
If we look at the living cell of an Amoeba or a Spirogyra, we see a something which exhibits certain active movements, and a certain fluctuating, or more or less lasting, form; and its form at a given moment, just like its motions, is to be investigated by the help of physical methods, and explained by the invocation of the mathematical conception of force.
Now the state, including the shape or form, of a portion of matter, is the resultant of a number of forces, which represent or symbolise the manifestations of various kinds of energy; and it is obvious, accordingly, that a great part of physical science must be understood or taken for granted as the necessary preliminary to the discussion on which we are engaged. But we may at least try to indicate, very briefly, the nature of the principal forces and the principal properties of matter with which our subject obliges us to deal. Let us imagine, for instance, the case of a so-called “simple” organism, such as Amoeba; and if our short list of its physical properties and conditions be helpful to our further discussion, we need not consider how far it be complete or adequate from the wider physical point of view23.
This portion of matter, then, is kept together by the intermolecular force of cohesion; in the movements of its particles relatively to one another, and in its own movements relative to adjacent matter, it meets with the opposing force of friction. It is acted on by gravity, and this force tends (though slightly, owing to the Amoeba’s small mass, and to the small difference between its density and that of the surrounding fluid), to flatten it down upon the solid substance on which it may be creeping. Our Amoeba tends, in the next place, to be deformed by any pressure from outside, even though slight, which may be applied to it, and this circumstance shews it to consist of matter in a fluid, or at least semi-fluid, state: which state is further indicated when we observe streaming or current motions in its interior. {13} Like other fluid bodies, its surface, whatsoever other substance, gas, liquid or solid, it be in contact with, and in varying degree according to the nature of that adjacent substance, is the seat of molecular force exhibiting itself as a surface-tension, from the action of which many important consequences follow, which greatly affect the form of the fluid surface.
While the protoplasm of the Amoeba reacts to the slightest pressure, and tends to “flow,” and while we therefore speak of it as a fluid, it is evidently far less mobile than such a fluid, for instance, as water, but is rather like treacle in its slow creeping movements as it changes its shape in response to force. Such fluids are said to have a high viscosity, and this viscosity obviously acts in the way of retarding change of form, or in other words of retarding the effects of any disturbing action of force. When the viscous fluid is capable of being drawn out into fine threads, a property in which we know that the material of some Amoebae differs greatly from that of others, we say that the fluid is also viscid, or exhibits viscidity. Again, not by virtue of our Amoeba being liquid, but at the same time in vastly greater measure than if it were a solid (though far less rapidly than if it were a gas), a process of molecular diffusion is constantly going on within its substance, by which its particles interchange their places within the mass, while surrounding fluids, gases and solids in solution diffuse into and out of it. In so far as the outer wall of the cell is different in character from the interior, whether it be a mere pellicle as in Amoeba or a firm cell-wall as in Protococcus, the diffusion which takes place through this wall is sometimes distinguished under the term osmosis.
Within the cell, chemical forces are at work, and so also in all probability (to judge by analogy) are electrical forces; and the organism reacts also to forces from without, that have their origin in chemical, electrical and thermal influences. The processes of diffusion and of chemical activity within the cell result, by the drawing in of water, salts, and food-material with or without chemical transformation into protoplasm, in growth, and this complex phenomenon we shall usually, without discussing its nature and origin, describe and picture as a force. Indeed we shall manifestly be inclined to use the term growth in two senses, {14} just indeed as we do in the case of attraction or gravitation, on the one hand as a process, and on the other hand as a force.
In the phenomena of cell-division, in the attractions or repulsions of the parts of the dividing nucleus and in the “caryokinetic” figures that appear in connection with it, we seem to see in operation forces and the effects of forces, that have, to say the least of it, a close analogy with known physical phenomena; and to this matter we shall afterwards recur. But though they resemble known physical phenomena, their nature is still the subject of much discussion, and neither the forms produced nor the forces at work can yet be satisfactorily and simply explained. We may readily admit, then, that besides phenomena which are obviously physical in their nature, there are actions visible as well as invisible taking place within living cells which our knowledge does not permit us to ascribe with certainty to any known physical force; and it may or may not be that these phenomena will yield in time to the methods of physical investigation. Whether or no, it is plain that we have no clear rule or guide as to what is “vital” and what is not; the whole assemblage of so-called vital phenomena, or properties of the organism, cannot be clearly classified into those that are physical in origin and those that are sui generis and peculiar to living things. All we can do meanwhile is to analyse, bit by bit, those parts of the whole to which the ordinary laws of the physical forces more or less obviously and clearly and indubitably apply.
Morphology then is not only a study of material things and of the forms of material things, but has its dynamical aspect, under which we deal with the interpretation, in terms of force, of the operations of Energy. And here it is well worth while to remark that, in dealing with the facts of embryology or the phenomena of inheritance, the common language of the books seems to deal too much with the material elements concerned, as the causes of development, of variation or of hereditary transmission. Matter as such produces nothing, changes nothing, does nothing; and however convenient it may afterwards be to abbreviate our nomenclature and our descriptions, we must most carefully realise in the outset that the spermatozoon, the nucleus, {15} the chromosomes or the germ-plasm can never act as matter alone, but only as seats of energy and as centres of force. And this is but an adaptation (in the light, or rather in the conventional symbolism, of modern physical science) of the old saying of the philosopher: ἀρχὴ γὰρ ἡ φύσις μᾶλλον τῆς ὕλης.
1 These sayings of Kant and of Du Bois, and others like to them, have been the text of many discourses: see, for instance, Stallo’s Concepts, p. 21, 1882; Höber, Biol. Centralbl. XIX, p. 284, 1890, etc. Cf. also Jellett, Rep. Brit. Ass. 1874, p. 1.
2 “Quum enim mundi universi fabrica sit perfectissima, atque a Creatore sapientissimo absoluta, nihil omnino in mundo contingit in quo non maximi minimive ratio quaepiam eluceat; quamobrem dubium prorsus est nullum quin omnes mundi effectus ex causis finalibus, ope methodi maximorum et minimorum, aeque feliciter determinari queant atque ex ipsis causis efficientibus.” Methodus inveniendi, etc. 1744 (cit. Mach, Science of Mechanics, 1902, p. 455).
3 Cf. Opp. (ed. Erdmann), p. 106, “Bien loin d’exclure les causes finales..., c’est de là qu’il faut tout déduire en Physique.”
4 Cf. p. 162. “La force vitale dirige des phénomènes qu’elle ne produit pas: les agents physiques produisent des phénomènes qu’ils ne dirigent pas.”
5 It is now and then conceded with reluctance. Thus Enriques, a learned and philosophic naturalist, writing “della economia di sostanza nelle osse cave” (Arch. f. Entw. Mech. XX, 1906), says “una certa impronta di teleologismo quà e là è rimasta, mio malgrado, in questo scritto.”
6 Cf. Cleland, On Terminal Forms of Life, J. Anat. and Phys. XVIII, 1884.
7 Conklin, Embryology of Crepidula, Journ. of Morphol. XIII, p. 203, 1897; Lillie, F. R., Adaptation in Cleavage, Woods Holl Biol. Lectures, pp. 43–67, 1899.
8 I am inclined to trace back Driesch’s teaching of Entelechy to no less a person than Melanchthon. When Bacon (de Augm. IV, 3) states with disapproval that the soul “has been regarded rather as a function than as a substance,” R. L. Ellis points out that he is referring to Melanchthon’s exposition of the Aristotelian doctrine. For Melanchthon, whose view of the peripatetic philosophy had long great influence in the Protestant Universities, affirmed that, according to the true view of Aristotle’s opinion, the soul is not a substance, but an ἑντελέχεια, or function. He defined it as δύναμις quaedam ciens actiones—a description all but identical with that of Claude Bernard’s “force vitale.”
9 Ray Lankester, Encycl. Brit. (9th ed.), art. “Zoology,” p. 806, 1888.
10 Alfred Russel Wallace, especially in his later years, relied upon a direct but somewhat crude teleology. Cf. his World of Life, a Manifestation of Creative Power, Directive Mind and Ultimate Purpose, 1910.
11 Janet, Les Causes Finales, 1876, p. 350.
12 The phrase is Leibniz’s, in his Théodicée.
13 Cf. (int. al.) Bosanquet, The Meaning of Teleology, Proc. Brit. Acad. 1905–6, pp. 235–245. Cf. also Leibniz (Discours de Métaphysique; Lettres inédites, ed. de Careil, 1857, p. 354; cit. Janet, p. 643), “L’un et l’autre est bon, l’un et l’autre peut être utile ... et les auteurs qui suivent ces routes différentes ne devraient point se maltraiter: et seq.”
14 The reader will understand that I speak, not of the “severe and diligent inquiry” of variation or of “fortuity,” but merely of the easy assumption that these phenomena are a sufficient basis on which to rest, with the all-powerful help of natural selection, a theory of definite and progressive evolution.
15 Revue Philosophique. XXXIII, 1892.
16 This general principle was clearly grasped by Dr George Rainey (a learned physician of St Bartholomew’s) many years ago, and expressed in such words as the following: “......it is illogical to suppose that in the case of vital organisms a distinct force exists to produce results perfectly within the reach of physical agencies, especially as in many instances no end could be attained were that the case, but that of opposing one force by another capable of effecting exactly the same purpose.” (On Artificial Calculi, Q.J.M.S. (Trans. Microsc. Soc.), VI, p. 49, 1858.) Cf. also Helmholtz, infra cit., p. 9.
17 Whereby he incurred the reproach of Socrates, in the Phaedo.
18 In a famous lecture (Conservation of Forces applied to Organic Nature, Proc. Roy. Instit., April 12, 1861), Helmholtz laid it down, as “the fundamental principle of physiology,” that “There may be other agents acting in the living body than those agents which act in the inorganic world; but those forces, as far as they cause chemical and mechanical influence in the body, must be quite of the same character as inorganic forces: in this at least, that their effects must be ruled by necessity, and must always be the same when acting in the same conditions; and so there cannot exist any arbitrary choice in the direction of their actions.” It would follow from this, that, like the other “physical” forces, they must be subject to mathematical analysis and deduction. Cf. also Dr T. Young’s Croonian Lecture On the Heart and Arteries, Phil. Trans. 1809, p. 1; Coll. Works, I, 511.
19 Ektropismus, oder die physikalische Theorie des Lebens, Leipzig, 1910.
20 Wilde Lecture, Nature, March 12, 1908; ibid. Sept. 6, 1900, p. 485; Aether and Matter, p. 288. Cf. also Lord Kelvin, Fortnightly Review, 1892, p. 313.
21 Joly, The Abundance of Life, Proc. Roy. Dublin Soc. VII, 1890; and in Scientific Essays, etc. 1915, p. 60 et seq.
22 Papillon, Histoire de la philosophie moderne, I, p. 300.
23 With the special and important properties of colloidal matter we are, for the time being, not concerned.
CHAPTER II. ON MAGNITUDE
To terms of magnitude, and of direction, must we refer all our conceptions of form. For the form of an object is defined when we know its magnitude, actual or relative, in various directions; and growth involves the same conceptions of magnitude and direction, with this addition, that they are supposed to alter in time. Before we proceed to the consideration of specific form, it will be worth our while to consider, for a little while, certain phenomena of spatial magnitude, or of the extension of a body in the several dimensions of space24.
We are taught by elementary mathematics that, in similar solid figures, the surface increases as the square, and the volume as the cube, of the linear dimensions. If we take the simple case of a sphere, with radius r, the area of its surface is equal to 4πr2 , and its volume to (4⁄3)πr3 ; from which it follows that the ratio of volume to surface, or V⁄S , is (1⁄3)r. In other words, the greater the radius (or the larger the sphere) the greater will be its volume, or its mass (if it be uniformly dense throughout), in comparison with its superficial area. And, taking L to represent any linear dimension, we may write the general equations in the form
S ∝ L2 , V ∝ L3 ,
or
S = k · L2 , and V = k′ · L3 ;
and
V⁄S ∝ L.
From these elementary principles a great number of consequences follow, all more or less interesting, and some of them of great importance. In the first place, though growth in length (let {17} us say) and growth in volume (which is usually tantamount to mass or weight) are parts of one and the same process or phenomenon, the one attracts our attention by its increase, very much more than the other. For instance a fish, in doubling its length, multiplies its weight by no less than eight times; and it all but doubles its weight in growing from four inches long to five.
In the second place we see that a knowledge of the correlation between length and weight in any particular species of animal, in other words a determination of k in the formula W = k · L3 , enables us at any time to translate the one magnitude into the other, and (so to speak) to weigh the animal with a measuring-rod; this however being always subject to the condition that the animal shall in no way have altered its form, nor its specific gravity. That its specific gravity or density should materially or rapidly alter is not very likely; but as long as growth lasts, changes of form, even though inappreciable to the eye, are likely to go on. Now weighing is a far easier and far more accurate operation than measuring; and the measurements which would reveal slight and otherwise imperceptible changes in the form of a fish—slight relative differences between length, breadth and depth, for instance,—would need to be very delicate indeed. But if we can make fairly accurate determinations of the length, which is very much the easiest dimension to measure, and then correlate it with the weight, then the value of k, according to whether it varies or remains constant, will tell us at once whether there has or has not been a tendency to gradual alteration in the general form. To this subject we shall return, when we come to consider more particularly the rate of growth.
But a much deeper interest arises out of this changing ratio of dimensions when we come to consider the inevitable changes of physical relations with which it is bound up. We are apt, and even accustomed, to think that magnitude is so purely relative that differences of magnitude make no other or more essential difference; that Lilliput and Brobdingnag are all alike, according as we look at them through one end of the glass or the other. But this is by no means so; for scale has a very marked effect upon physical phenomena, and the effect of scale constitutes what is known as the principle of similitude, or of dynamical similarity. {18}
This effect of scale is simply due to the fact that, of the physical forces, some act either directly at the surface of a body, or otherwise in proportion to the area of surface; and others, such as gravity, act on all particles, internal and external alike, and exert a force which is proportional to the mass, and so usually to the volume, of the body.
The strength of an iron girder obviously varies with the cross-section of its members, and each cross-section varies as the square of a linear dimension; but the weight of the whole structure varies as the cube of its linear dimensions. And it follows at once that, if we build two bridges geometrically similar, the larger is the weaker of the two25. It was elementary engineering experience such as this that led Herbert Spencer26 to apply the principle of similitude to biology.
The same principle had been admirably applied, in a few clear instances, by Lesage27, a celebrated eighteenth century physician of Geneva, in an unfinished and unpublished work28. Lesage argued, for instance, that the larger ratio of surface to mass would lead in a small animal to excessive transpiration, were the skin as “porous” as our own; and that we may hence account for the hardened or thickened skins of insects and other small terrestrial animals. Again, since the weight of a fruit increases as the cube of its dimensions, while the strength of the stalk increases as the square, it follows that the stalk should grow out of apparent due proportion to the fruit; or alternatively, that tall trees should not bear large fruit on slender branches, and that melons and pumpkins must lie upon the ground. And again, that in quadrupeds a large head must be supported on a neck which is either {19} excessively thick and strong, like a bull’s, or very short like the neck of an elephant.
But it was Galileo who, wellnigh 300 years ago, had first laid down this general principle which we now know by the name of the principle of similitude; and he did so with the utmost possible clearness, and with a great wealth of illustration, drawn from structures living and dead29. He showed that neither can man build a house nor can nature construct an animal beyond a certain size, while retaining the same proportions and employing the same materials as sufficed in the case of a smaller structure30. The thing will fall to pieces of its own weight unless we either change its relative proportions, which will at length cause it to become clumsy, monstrous and inefficient, or else we must find a new material, harder and stronger than was used before. Both processes are familiar to us in nature and in art, and practical applications, undreamed of by Galileo, meet us at every turn in this modern age of steel.
Again, as Galileo was also careful to explain, besides the questions of pure stress and strain, of the strength of muscles to lift an increasing weight or of bones to resist its crushing stress, we have the very important question of bending moments. This question enters, more or less, into our whole range of problems; it affects, as we shall afterwards see, or even determines the whole form of the skeleton, and is very important in such a case as that of a tall tree31.
Here we have to determine the point at which the tree will curve under its own weight, if it be ever so little displaced from the perpendicular32. In such an investigation we have to make {20} some assumptions,—for instance, with regard to the trunk, that it tapers uniformly, and with regard to the branches that their sectional area varies according to some definite law, or (as Ruskin assumed33) tends to be constant in any horizontal plane; and the mathematical treatment is apt to be somewhat difficult. But Greenhill has shewn that (on such assumptions as the above), a certain British Columbian pine-tree, which yielded the Kew flagstaff measuring 221 ft. in height with a diameter at the base of 21 inches, could not possibly, by theory, have grown to more than about 300 ft. It is very curious that Galileo suggested precisely the same height (dugento braccia alta) as the utmost limit of the growth of a tree. In general, as Greenhill shews, the diameter of a homogeneous body must increase as the power 3 ⁄ 2 of the height, which accounts for the slender proportions of young trees, compared with the stunted appearance of old and large ones34. In short, as Goethe says in Wahrheit und Dichtung, “Es ist dafür gesorgt dass die Bäume nicht in den Himmel wachsen.” But Eiffel’s great tree of steel (1000 feet high) is built to a very different plan; for here the profile of the tower follows the logarithmic curve, giving equal strength throughout, according to a principle which we shall have occasion to discuss when we come to treat of “form and mechanical efficiency” in connection with the skeletons of animals.
Among animals, we may see in a general way, without the help of mathematics or of physics, that exaggerated bulk brings with it a certain clumsiness, a certain inefficiency, a new element of risk and hazard, a vague preponderance of disadvantage. The case was well put by Owen, in a passage which has an interest of its own as a premonition (somewhat like De Candolle’s) of the “struggle for existence.” Owen wrote as follows35: “In proportion to the bulk of a species is the difficulty of the contest which, as a living organised whole, the individual of such species {21} has to maintain against the surrounding agencies that are ever tending to dissolve the vital bond, and subjugate the living matter to the ordinary chemical and physical forces. Any changes, therefore, in such external conditions as a species may have been originally adapted to exist in, will militate against that existence in a degree proportionate, perhaps in a geometrical ratio, to the bulk of the species. If a dry season be greatly prolonged, the large mammal will suffer from the drought sooner than the small one; if any alteration of climate affect the quantity of vegetable food, the bulky Herbivore will first feel the effects of stinted nourishment.”
But the principle of Galileo carries us much further and along more certain lines.
The tensile strength of a muscle, like that of a rope or of our girder, varies with its cross-section; and the resistance of a bone to a crushing stress varies, again like our girder, with its cross-section. But in a terrestrial animal the weight which tends to crush its limbs or which its muscles have to move, varies as the cube of its linear dimensions; and so, to the possible magnitude of an animal, living under the direct action of gravity, there is a definite limit set. The elephant, in the dimensions of its limb-bones, is already shewing signs of a tendency to disproportionate thickness as compared with the smaller mammals; its movements are in many ways hampered and its agility diminished: it is already tending towards the maximal limit of size which the physical forces permit. But, as Galileo also saw, if the animal be wholly immersed in water, like the whale, (or if it be partly so, as was in all probability the case with the giant reptiles of our secondary rocks), then the weight is counterpoised to the extent of an equivalent volume of water, and is completely counterpoised if the density of the animal’s body, with the included air, be identical (as in a whale it very nearly is) with the water around. Under these circumstances there is no longer a physical barrier to the indefinite growth in magnitude of the animal36. Indeed, {22} in the case of the aquatic animal there is, as Spencer pointed out, a distinct advantage, in that the larger it grows the greater is its velocity. For its available energy depends on the mass of its muscles; while its motion through the water is opposed, not by gravity, but by “skin-friction,” which increases only as the square of its dimensions; all other things being equal, the bigger the ship, or the bigger the fish, the faster it tends to go, but only in the ratio of the square root of the increasing length. For the mechanical work (W) of which the fish is capable being proportional to the mass of its muscles, or the cube of its linear dimensions: and again this work being wholly done in producing a velocity (V) against a resistance (R) which increases as the square of the said linear dimensions; we have at once
W = l3 ,
and also
W = RV2 = l2V2 .
Therefore
l3 = l2V2 , and V = √l.
This is what is known as Froude’s Law of the correspondence of speeds.
But there is often another side to these questions, which makes them too complicated to answer in a word. For instance, the work (per stroke) of which two similar engines are capable should obviously vary as the cubes of their linear dimensions, for it varies on the one hand with the surface of the piston, and on the other, with the length of the stroke; so is it likewise in the animal, where the corresponding variation depends on the cross-section of the muscle, and on the space through which it contracts. But in two precisely similar engines, the actual available horse-power varies as the square of the linear dimensions, and not as the cube; and this for the obvious reason that the actual energy developed depends upon the heating-surface of the boiler37. So likewise must there be a similar tendency, among animals, for the rate of supply of kinetic energy to vary with the surface of the {23} lung, that is to say (other things being equal) with the square of the linear dimensions of the animal. We may of course (departing from the condition of similarity) increase the heating-surface of the boiler, by means of an internal system of tubes, without increasing its outward dimensions, and in this very way nature increases the respiratory surface of a lung by a complex system of branching tubes and minute air-cells; but nevertheless in two similar and closely related animals, as also in two steam-engines of precisely the same make, the law is bound to hold that the rate of working must tend to vary with the square of the linear dimensions, according to Froude’s law of steamship comparison. In the case of a very large ship, built for speed, the difficulty is got over by increasing the size and number of the boilers, till the ratio between boiler-room and engine-room is far beyond what is required in an ordinary small vessel38; but though we find lung-space increased among animals where greater rate of working is required, as in general among birds, I do not know that it can be shewn to increase, as in the “over-boilered” ship, with the size of the animal, and in a ratio which outstrips that of the other bodily dimensions. If it be the case then, that the working mechanism of the muscles should be able to exert a force proportionate to the cube of the linear bodily dimensions, while the respiratory mechanism can only supply a store of energy at a rate proportional to the square of the said dimensions, the singular result ought to follow that, in swimming for instance, the larger fish ought to be able to put on a spurt of speed far in excess of the smaller one; but the distance travelled by the year’s end should be very much alike for both of them. And it should also follow that the curve of fatigue {24} should be a steeper one, and the staying power should be less, in the smaller than in the larger individual. This is the case of long-distance racing, where the big winner puts on his big spurt at the end. And for an analogous reason, wise men know that in the ’Varsity boat-race it is judicious and prudent to bet on the heavier crew.
Leaving aside the question of the supply of energy, and keeping to that of the mechanical efficiency of the machine, we may find endless biological illustrations of the principle of similitude.
In the case of the flying bird (apart from the initial difficulty of raising itself into the air, which involves another problem) it may be shewn that the bigger it gets (all its proportions remaining the same) the more difficult it is for it to maintain itself aloft in flight. The argument is as follows:
In order to keep aloft, the bird must communicate to the air a downward momentum equivalent to its own weight, and therefore proportional to the cube of its own linear dimensions. But the momentum so communicated is proportional to the mass of air driven downwards, and to the rate at which it is driven: the mass being proportional to the bird’s wing-area, and also (with any given slope of wing) to the speed of the bird, and the rate being again proportional to the bird’s speed; accordingly the whole momentum varies as the wing-area, i.e. as the square of the linear dimensions, and also as the square of the speed. Therefore, in order that the bird may maintain level flight, its speed must be proportional to the square root of its linear dimensions.
Now the rate at which the bird, in steady flight, has to work in order to drive itself forward, is the rate at which it communicates energy to the air; and this is proportional to mV2 , i.e. to the mass and to the square of the velocity of the air displaced. But the mass of air displaced per second is proportional to the wing-area and to the speed of the bird’s motion, and therefore to the power 2½ of the linear dimensions; and the speed at which it is displaced is proportional to the bird’s speed, and therefore to the square root of the linear dimensions. Therefore the energy communicated per second (being proportional to the mass and to the square of the speed) is jointly proportional to the power 2½ of the linear dimensions, as above, and to the first power thereof: {25} that is to say, it increases in proportion to the power 3½ of the linear dimensions, and therefore faster than the weight of the bird increases.
Put in mathematical form, the equations are as follows:
(m = the mass of air thrust downwards; V its velocity, proportional to that of the bird; M its momentum; l a linear dimension of the bird; w its weight; W the work done in moving itself forward.)
M = w = l3 .
But
M = m V, and m = l2 V.
Therefore
M = l2 V2 , and
l2 V2 = l3 , or
V = √l.
But, again,
W = m V2
= l2 V × V2
= l2 × √l × l
= l3½ .
The work requiring to be done, then, varies as the power 3½ of the bird’s linear dimensions, while the work of which the bird is capable depends on the mass of its muscles, and therefore varies as the cube of its linear dimensions39. The disproportion does not seem at first sight very great, but it is quite enough to tell. It is as much as to say that, every time we double the linear dimensions of the bird, the difficulty of flight is increased in the ratio of 23 : 23½ , or 8 : 11·3, or, say, 1 : 1·4. If we take the ostrich to exceed the sparrow in linear dimensions as 25 : 1, which seems well within the mark, we have the ratio between 253½ and 253 , or between 57 : 56 ; in other words, flight is just five times more difficult for the larger than for the smaller bird40.
The above investigation includes, besides the final result, a number of others, explicit or implied, which are of not less importance. Of these the simplest and also the most important is {26} contained in the equation V = √l, a result which happens to be identical with one we had also arrived at in the case of the fish. In the bird’s case it has a deeper significance than in the other; because it implies here not merely that the velocity will tend to increase in a certain ratio with the length, but that it must do so as an essential and primary condition of the bird’s remaining aloft. It is accordingly of great practical importance in aeronautics, for it shews how a provision of increasing speed must accompany every enlargement of our aeroplanes. If a given machine weighing, say, 500 lbs. be stable at 40 miles an hour, then one geometrically similar which weighs, say, a couple of tons must have its speed determined as follows:
W : w :: L3 : l3 :: 8 : 1.
Therefore
L : l :: 2 : 1.
But
V2 : v2 :: L : l.
Therefore
V : v :: √2 : 1 = 1·414 : 1.
That is to say, the larger machine must be capable of a speed equal to 1·414 × 40, or about 56½ miles per hour.
It is highly probable, as Lanchester41 remarks, that Lilienthal met his untimely death not so much from any intrinsic fault in the design or construction of his machine, but simply because his engine fell somewhat short of the power required to give the speed which was necessary for stability. An arrow is a very imperfectly designed aeroplane, but nevertheless it is evidently capable, to a certain extent and at a high velocity, of acquiring “stability” and hence of actual “flight”: the duration and consequent range of its trajectory, as compared with a bullet of similar initial velocity, being correspondingly benefited. When we return to our birds, and again compare the ostrich with the sparrow, we know little or nothing about the speed in flight of the latter, but that of the swift is estimated42 to vary from a minimum of 20 to 50 feet or more per second,—say from 14 to 35 miles per hour. Let us take the same lower limit as not far from the minimal velocity of the sparrow’s flight also; and it {27} would follow that the ostrich, of 25 times the sparrow’s linear dimensions, would be compelled to fly (if it flew at all) with a minimum velocity of 5 × 14, or 70 miles an hour.
The same principle of necessary speed, or the indispensable relation between the dimensions of a flying object and the minimum velocity at which it is stable, accounts for a great number of observed phenomena. It tells us why the larger birds have a marked difficulty in rising from the ground, that is to say, in acquiring to begin with the horizontal velocity necessary for their support; and why accordingly, as Mouillard43 and others have observed, the heavier birds, even those weighing no more than a pound or two, can be effectively “caged” in a small enclosure open to the sky. It tells us why very small birds, especially those as small as humming-birds, and à fortiori the still smaller insects, are capable of “stationary flight,” a very slight and scarcely perceptible velocity relatively to the air being sufficient for their support and stability. And again, since it is in all cases velocity relative to the air that we are speaking of, we comprehend the reason why one may always tell which way the wind blows by watching the direction in which a bird starts to fly.
It is not improbable that the ostrich has already reached a magnitude, and we may take it for certain that the moa did so, at which flight by muscular action, according to the normal anatomy of a bird, has become physiologically impossible. The same reasoning applies to the case of man. It would be very difficult, and probably absolutely impossible, for a bird to fly were it the bigness of a man. But Borelli, in discussing this question, laid even greater stress on the obvious fact that a man’s pectoral muscles are so immensely less in proportion than those of a bird, that however we may fit ourselves with wings we can never expect to move them by any power of our own relatively weaker muscles; so it is that artificial flight only became possible when an engine was devised whose efficiency was extraordinarily great in comparison with its weight and size.
Had Leonardo da Vinci known what Galileo knew, he would not have spent a great part of his life on vain efforts to make to himself wings. Borelli had learned the lesson thoroughly, and {28} in one of his chapters he deals with the proposition, “Est impossible, ut homines propriis viribus artificiose volare possint44.”
But just as it is easier to swim than to fly, so is it obvious that, in a denser atmosphere, the conditions of flight would be altered, and flight facilitated. We know that in the carboniferous epoch there lived giant dragon-flies, with wings of a span far greater than nowadays they ever attain; and the small bodies and huge extended wings of the fossil pterodactyles would seem in like manner to be quite abnormal according to our present standards, and to be beyond the limits of mechanical efficiency under present conditions. But as Harlé suggests45, following upon a suggestion of Arrhenius, we have only to suppose that in carboniferous and jurassic days the terrestrial atmosphere was notably denser than it is at present, by reason, for instance, of its containing a much larger proportion of carbonic acid, and we have at once a means of reconciling the apparent mechanical discrepancy.
Very similar problems, involving in various ways the principle of dynamical similitude, occur all through the physiology of locomotion: as, for instance, when we see that a cockchafer can carry a plate, many times his own weight, upon his back, or that a flea can jump many inches high.
Problems of this latter class have been admirably treated both by Galileo and by Borelli, but many later writers have remained ignorant of their work. Linnaeus, for instance, remarked that, if an elephant were as strong in proportion as a stag-beetle, it would be able to pull up rocks by the root, and to level mountains. And Kirby and Spence have a well-known passage directed to shew that such powers as have been conferred upon the insect have been withheld from the higher animals, for the reason that had these latter been endued therewith they would have “caused the early desolation of the world46.” {29}
Such problems as that which is presented by the flea’s jumping powers, though essentially physiological in their nature, have their interest for us here: because a steady, progressive diminution of activity with increasing size would tend to set limits to the possible growth in magnitude of an animal just as surely as those factors which tend to break and crush the living fabric under its own weight. In the case of a leap, we have to do rather with a sudden impulse than with a continued strain, and this impulse should be measured in terms of the velocity imparted. The velocity is proportional to the impulse (x), and inversely proportional to the mass (M) moved: V = x ⁄ M. But, according to what we still speak of as “Borelli’s law,” the impulse (i.e. the work of the impulse) is proportional to the volume of the muscle by which it is produced47, that is to say (in similarly constructed animals) to the mass of the whole body; for the impulse is proportional on the one hand to the cross-section of the muscle, and on the other to the distance through which it contracts. It follows at once from this that the velocity is constant, whatever be the size of the animals: in other words, that all animals, provided always that they are similarly fashioned, with their various levers etc., in like proportion, ought to jump, not to the same relative, but to the same actual height48. According to this, then, the flea is not a better, but rather a worse jumper than a horse or a man. As a matter of fact, Borelli is careful to point out that in the act of leaping the impulse is not actually instantaneous, as in the blow of a hammer, but takes some little time, during which the levers are being extended by which the centre of gravity of the animal is being propelled forwards; and this interval of time will be longer in the case of the longer levers of the larger animal. To some extent, then, this principle acts as a corrective to the more general one, {30} and tends to leave a certain balance of advantage, in regard to leaping power, on the side of the larger animal49.
But on the other hand, the question of strength of materials comes in once more, and the factors of stress and strain and bending moment make it, so to speak, more and more difficult for nature to endow the larger animal with the length of lever with which she has provided the flea or the grasshopper.
To Kirby and Spence it seemed that “This wonderful strength of insects is doubtless the result of something peculiar in the structure and arrangement of their muscles, and principally their extraordinary power of contraction.” This hypothesis, which is so easily seen, on physical grounds, to be unnecessary, has been amply disproved in a series of excellent papers by F. Plateau50.
A somewhat simple problem is presented to us by the act of walking. It is obvious that there will be a great economy of work, if the leg swing at its normal pendulum-rate; and, though this rate is hard to calculate, owing to the shape and the jointing of the limb, we may easily convince ourselves, by counting our steps, that the leg does actually swing, or tend to swing, just as a pendulum does, at a certain definite rate51. When we walk quicker, we cause the leg-pendulum to describe a greater arc, but we do not appreciably cause it to swing, or vibrate, quicker, until we shorten the pendulum and begin to run. Now let two individuals, A and B, walk in a similar fashion, that is to say, with a similar angle of swing. The arc through which the leg swings, or the amplitude of each step, will therefore vary as the length of leg, or say as a ⁄ b; but the time of swing will vary as the square {31} root of the pendulum-length, or √a ⁄ √b. Therefore the velocity, which is measured by amplitude ⁄ time, will also vary as the square-roots of the length of leg: that is to say, the average velocities of A and B are in the ratio of √a : √b.
The smaller man, or smaller animal, is so far at a disadvantage compared with the larger in speed, but only to the extent of the ratio between the square roots of their linear dimensions: whereas, if the rate of movement of the limb were identical, irrespective of the size of the animal,—if the limbs of the mouse for instance swung at the same rate as those of the horse,—then, as F. Plateau said, the mouse would be as slow or slower in its gait than the tortoise. M. Delisle52 observed a “minute fly” walk three inches in half-a-second. This was good steady walking. When we walk five miles an hour we go about 88 inches in a second, or 88 ⁄ 6 = 14·7 times the pace of M. Delisle’s fly. We should walk at just about the fly’s pace if our stature were 1 ⁄ (14·7)2 , or 1 ⁄ 216 of our present height,—say 72 ⁄ 216 inches, or one-third of an inch high.
But the leg comprises a complicated system of levers, by whose various exercise we shall obtain very different results. For instance, by being careful to rise upon our instep, we considerably increase the length or amplitude of our stride, and very considerably increase our speed accordingly. On the other hand, in running, we bend and so shorten the leg, in order to accommodate it to a quicker rate of pendulum-swing53. In short, the jointed structure of the leg permits us to use it as the shortest possible pendulum when it is swinging, and as the longest possible lever when it is exerting its propulsive force.
Apart from such modifications as that described in the last paragraph,—apart, that is to say, from differences in mechanical construction or in the manner in which the mechanism is used,—we have now arrived at a curiously simple and uniform result. For in all the three forms of locomotion which we have attempted {32} to study, alike in swimming, in flight and in walking, the general result, attained under very different conditions and arrived at by very different modes of reasoning, is in every case that the velocity tends to vary as the square root of the linear dimensions of the organism.
From all the foregoing discussion we learn that, as Crookes once upon a time remarked54, the form as well as the actions of our bodies are entirely conditioned (save for certain exceptions in the case of aquatic animals, nicely balanced with the density of the surrounding medium) by the strength of gravity upon this globe. Were the force of gravity to be doubled, our bipedal form would be a failure, and the majority of terrestrial animals would resemble short-legged saurians, or else serpents. Birds and insects would also suffer, though there would be some compensation for them in the increased density of the air. While on the other hand if gravity were halved, we should get a lighter, more graceful, more active type, requiring less energy and less heat, less heart, less lungs, less blood.
Throughout the whole field of morphology we may find examples of a tendency (referable doubtless in each case to some definite physical cause) for surface to keep pace with volume, through some alteration of its form. The development of “villi” on the inner surface of the stomach and intestine (which enlarge its surface much as we enlarge the effective surface of a bath-towel), the various valvular folds of the intestinal lining, including the remarkable “spiral fold” of the shark’s gut, the convolutions of the brain, whose complexity is evidently correlated (in part at least) with the magnitude of the animal,—all these and many more are cases in which a more or less constant ratio tends to be maintained between mass and surface, which ratio would have been more and more departed from had it not been for the alterations of surface-form55. {33}
In the case of very small animals, and of individual cells, the principle becomes especially important, in consequence of the molecular forces whose action is strictly limited to the superficial layer. In the cases just mentioned, action is facilitated by increase of surface: diffusion, for instance, of nutrient liquids or respiratory gases is rendered more rapid by the greater area of surface; but there are other cases in which the ratio of surface to mass may make an essential change in the whole condition of the system. We know, for instance, that iron rusts when exposed to moist air, but that it rusts ever so much faster, and is soon eaten away, if the iron be first reduced to a heap of small filings; this is a mere difference of degree. But the spherical surface of the raindrop and the spherical surface of the ocean (though both happen to be alike in mathematical form) are two totally different phenomena, the one due to surface-energy, and the other to that form of mass-energy which we ascribe to gravity. The contrast is still more clearly seen in the case of waves: for the little ripple, whose form and manner of propagation are governed by surface-tension, is found to travel with a velocity which is inversely as the square root of its length; while the ordinary big waves, controlled by gravitation, have a velocity directly proportional to the square root of their wave-length. In like manner we shall find that the form of all small organisms is largely independent of gravity, and largely if not mainly due to the force of surface-tension: either as the direct result of the continued action of surface tension on the semi-fluid body, or else as the result of its action at a prior stage of development, in bringing about a form which subsequent chemical changes have rendered rigid and lasting. In either case, we shall find a very great tendency in small organisms to assume either the spherical form or other simple forms related to ordinary inanimate surface-tension phenomena; which forms do not recur in the external morphology of large animals, or if they in part recur it is for other reasons. {34}
Now this is a very important matter, and is a notable illustration of that principle of similitude which we have already discussed in regard to several of its manifestations. We are coming easily to a conclusion which will affect the whole course of our argument throughout this book, namely that there is an essential difference in kind between the phenomena of form in the larger and the smaller organisms. I have called this book a study of Growth and Form, because in the most familiar illustrations of organic form, as in our own bodies for example, these two factors are inseparably associated, and because we are here justified in thinking of form as the direct resultant and consequence of growth: of growth, whose varying rate in one direction or another has produced, by its gradual and unequal increments, the successive stages of development and the final configuration of the whole material structure. But it is by no means true that form and growth are in this direct and simple fashion correlative or complementary in the case of minute portions of living matter. For in the smaller organisms, and in the individual cells of the larger, we have reached an order of magnitude in which the intermolecular forces strive under favourable conditions with, and at length altogether outweigh, the force of gravity, and also those other forces leading to movements of convection which are the prevailing factors in the larger material aggregate.
However we shall require to deal more fully with this matter in our discussion of the rate of growth, and we may leave it meanwhile, in order to deal with other matters more or less directly concerned with the magnitude of the cell.
The living cell is a very complex field of energy, and of energy of many kinds, surface-energy included. Now the whole surface-energy of the cell is by no means restricted to its outer surface; for the cell is a very heterogeneous structure, and all its protoplasmic alveoli and other visible (as well as invisible) heterogeneities make up a great system of internal surfaces, at every part of which one “phase” comes in contact with another “phase,” and surface-energy is accordingly manifested. But still, the external surface is a definite portion of the system, with a definite “phase” of its own, and however little we may know of the distribution of the total energy of the system, it is at least plain that {35} the conditions which favour equilibrium will be greatly altered by the changed ratio of external surface to mass which a change of magnitude, unaccompanied by change of form, produces in the cell. In short, however it may be brought about, the phenomenon of division of the cell will be precisely what is required to keep approximately constant the ratio between surface and mass, and to restore the balance between the surface-energy and the other energies of the system. When a germ-cell, for instance, divides or “segments” into two, it does not increase in mass; at least if there be some slight alleged tendency for the egg to increase in mass or volume during segmentation, it is very slight indeed, generally imperceptible, and wholly denied by some56. The development or growth of the egg from a one-celled stage to stages of two or many cells, is thus a somewhat peculiar kind of growth; it is growth which is limited to increase of surface, unaccompanied by growth in volume or in mass.
In the case of a soap-bubble, by the way, if it divide into two bubbles, the volume is actually diminished57 while the surface-area is greatly increased. This is due to a cause which we shall have to study later, namely to the increased pressure due to the greater curvature of the smaller bubbles.
An immediate and remarkable result of the principles just described is a tendency on the part of all cells, according to their kind, to vary but little about a certain mean size, and to have, in fact, certain absolute limitations of magnitude.
Sachs58 pointed out, in 1895, that there is a tendency for each nucleus to be only able to gather around itself a certain definite amount of protoplasm. Driesch59, a little later, found that, by artificial subdivision of the egg, it was possible to rear dwarf sea-urchin larvae, one-half, one-quarter, or even one-eighth of their {36} normal size; and that these dwarf bodies were composed of only a half, a quarter or an eighth of the normal number of cells. Similar observations have been often repeated and amply confirmed. For instance, in the development of Crepidula (a little American “slipper-limpet,” now much at home on our own oyster-beds), Conklin60 has succeeded in rearing dwarf and giant individuals, of which the latter may be as much as twenty-five times as big as the former. But nevertheless, the individual cells, of skin, gut, liver, muscle, and of all the other tissues, are just the same size in one as in the other,—in dwarf and in giant61. Driesch has laid particular stress upon this principle of a “fixed cell-size.”
We get an excellent, and more familiar illustration of the same principle in comparing the large brain-cells or ganglion-cells, both of the lower and of the higher animals62.
24 Cf. Hans Przibram, Anwendung elementarer Mathematik auf Biologische Probleme (in Roux’s Vorträge, Heft III), Leipzig, 1908, p. 10.
25 The subject is treated from an engineering point of view by Prof. James Thomson, Comparisons of Similar Structures as to Elasticity, Strength, and Stability, Trans. Inst. Engineers, Scotland, 1876 (Collected Papers, 1912, pp. 361–372), and by Prof. A. Barr, ibid. 1899; see also Rayleigh, Nature, April 22, 1915.
26 Cf. Spencer, The Form of the Earth, etc., Phil. Mag. XXX, pp. 194–6, 1847; also Principles of Biology, pt. II, ch. I, 1864 (p. 123, etc.).
27 George Louis Lesage (1724–1803), well known as the author of one of the few attempts to explain gravitation. (Cf. Leray, Constitution de la Matière, 1869; Kelvin, Proc. R. S. E. VII, p. 577, 1872, etc.; Clerk Maxwell, Phil. Trans. vol. 157, p. 50, 1867; art. “Atom,” Encycl. Brit. 1875, p. 46.)
28 Cf. Pierre Prévost, Notices de la vie et des écrits de Lesage, 1805; quoted by Janet, Causes Finales, app. III.
29 Discorsi e Dimostrazioni matematiche, intorno à due nuove scienze, attenenti alla Mecanica, ed ai Movimenti Locali: appresso gli Elzevirii, MDCXXXVIII. Opere, ed. Favaro, VIII, p. 169 seq. Transl. by Henry Crew and A. de Salvio, 1914, p. 130, etc. See Nature, June 17, 1915.
30 So Werner remarked that Michael Angelo and Bramanti could not have built of gypsum at Paris on the scale they built of travertin in Rome.
31 Sir G. Greenhill, Determination of the greatest height to which a Tree of given proportions can grow, Cambr. Phil. Soc. Pr. IV, p. 65, 1881, and Chree, ibid. VII, 1892. Cf. Poynting and Thomson’s Properties of Matter, 1907, p 99.
32 In like manner the wheat-straw bends over under the weight of the loaded ear, and the tip of the cat’s tail bends over when held upright,—not because they “possess flexibility,” but because they outstrip the dimensions within which stable equilibrium is possible in a vertical position. The kitten’s tail, on the other hand, stands up spiky and straight.
33 Modern Painters.
34 The stem of the giant bamboo may attain a height of 60 metres, while not more than about 40 cm. in diameter near its base, which dimensions are not very far short of the theoretical limits (A. J. Ewart, Phil. Trans. vol. 198, p. 71, 1906).
35 Trans. Zool. Soc. IV, 1850, p. 27.
36 It would seem to be a common if not a general rule that marine organisms, zoophytes, molluscs, etc., tend to be larger than the corresponding and closely related forms living in fresh water. While the phenomenon may have various causes, it has been attributed (among others) to the simple fact that the forces of growth are less antagonised by gravity in the denser medium (cf. Houssay, La Forme et la Vie, 1900, p. 815). The effect of gravity on outward form is illustrated, for instance, by the contrast between the uniformly upward branching of a sea-weed and the drooping curves of a shrub or tree.
37 The analogy is not a very strict one. We are not taking account, for instance, of a proportionate increase in thickness of the boiler-plates.
38 Let L be the length, S the (wetted) surface, T the tonnage, D the displacement (or volume) of a ship; and let it cross the Atlantic at a speed V. Then, in comparing two ships, similarly constructed but of different magnitudes, we know that L = V2 , S = L2 = V4 , D = T = L3 = V6 ; also R (resistance) = S · V2 = V6 ; H (horse-power) = R · V = V7 ; and the coal (C) necessary for the voyage = H ⁄ V = V6 . That is to say, in ordinary engineering language, to increase the speed across the Atlantic by 1 per cent. the ship’s length must be increased 2 per cent., her tonnage or displacement 6 per cent., her coal-consumpt also 6 per cent., her horse-power, and therefore her boiler-capacity, 7 per cent. Her bunkers, accordingly, keep pace with the enlargement of the ship, but her boilers tend to increase out of proportion to the space available.
39 This is the result arrived at by Helmholtz, Ueber ein Theorem geometrisch ähnliche Bewegungen flüssiger Körper betreffend, nebst Anwendung auf das Problem Luftballons zu lenken, Monatsber. Akad. Berlin, 1873, pp. 501–14. It was criticised and challenged (somewhat rashly) by K. Müllenhof, Die Grösse der Flugflächen, etc., Pflüger’s Archiv, XXXV, p. 407, XXXVI, p. 548, 1885.
40 Cf. also Chabrier, Vol des Insectes, Mém. Mus. Hist. Nat. Paris, VI–VIII, 1820–22.
41 Aerial Flight, vol. II (Aerodonetics), 1908, p. 150.
42 By Lanchester, op. cit. p. 131.
43 Cf. L’empire de l’air; ornithologie appliquée à l’aviation. 1881.
44 De Motu Animalium, I, prop. cciv, ed. 1685, p. 243.
45 Harlé, On Atmospheric Pressure in past Geological Ages, Bull. Geol. Soc. Fr. XI, pp. 118–121; or Cosmos, p. 30, July 8, 1911.
46 Introduction to Entomology, 1826, II, p. 190. K. and S., like many less learned authors, are fond of popular illustrations of the “wonders of Nature,” to the neglect of dynamical principles. They suggest, for instance, that if the white ant were as big as a man, its tunnels would be “magnificent cylinders of more than three hundred feet in diameter”; and that if a certain noisy Brazilian insect were as big as a man, its voice would be heard all the world over: “so that Stentor becomes a mute when compared with these insects!” It is an easy consequence of anthropomorphism, and hence a common characteristic of fairy-tales, to neglect the principle of dynamical, while dwelling on the aspect of geometrical, similarity.
47 I.e. the available energy of muscle, in ft.-lbs. per lb. of muscle, is the same for all animals: a postulate which requires considerable qualification when we are comparing very different kinds of muscle, such as the insect’s and the mammal’s.
48 Prop. clxxvii. Animalia minora et minus ponderosa majores saltus efficiunt respectu sui corporis, si caetera fuerint paria.
49 See also (int. al.), John Bernoulli, de Motu Musculorum, Basil., 1694; Chabry, Mécanisme du Saut, J. de l’Anat. et de la Physiol. XIX, 1883; Sur la longueur des membres des animaux sauteurs, ibid. XXI, p. 356, 1885; Le Hello, De l’action des organes locomoteurs, etc., ibid. XXIX, p. 65–93, 1893, etc.
50 Recherches sur la force absolue des muscles des Invertébrés, Bull. Acad. E. de Belgique (3), VI, VII, 1883–84; see also ibid. (2), XX, 1865, XXII, 1866; Ann. Mag. N. H. XVII, p. 139, 1866, XIX, p. 95, 1867. The subject was also well treated by Straus-Dürckheim, in his Considérations générales sur l’anatomie comparée des animaux articulés, 1828.
51 The fact that the limb tends to swing in pendulum-time was first observed by the brothers Weber (Mechanik der menschl. Gehwerkzeuge, Göttingen, 1836). Some later writers have criticised the statement (e.g. Fischer, Die Kinematik des Beinschwingens etc., Abh. math. phys. Kl. k. Sächs. Ges. XXV–XXVIII, 1899–1903), but for all that, with proper qualifications, it remains substantially true.
52 Quoted in Mr John Bishop’s interesting article in Todd’s Cyclopaedia, III, p. 443.
53 There is probably also another factor involved here: for in bending, and therefore shortening, the leg we bring its centre of gravity nearer to the pivot, that is to say, to the joint, and so the muscle tends to move it the more quickly.
54 Proc. Psychical Soc. XII, pp. 338–355, 1897.
55 For various calculations of the increase of surface due to histological and anatomical subdivision, see E. Babak, Ueber die Oberflächenentwickelung bei Organismen, Biol. Centralbl. XXX, pp. 225–239, 257–267, 1910. In connection with the physical theory of surface-energy, Wolfgang Ostwald has introduced the conception of specific surface, that is to say the ratio of surface to volume, or S ⁄ V. In a cube, V = l3 , and S = 6l2 ; therefore S ⁄ V = 6 ⁄ l. Therefore if the side l measure 6 cm., the ratio S ⁄ V = 1, and such a cube may be taken as our standard, or unit of specific surface. A human blood-corpuscle has, accordingly, a specific surface of somewhere about 14,000 or 15,000. It is found in physical chemistry that surface energy becomes an important factor when the specific surface reaches a value of 10,000 or thereby.
56 Though the entire egg is not increasing in mass, this is not to say that its living protoplasm is not increasing all the while at the expense of the reserve material.
57 Cf. Tait, Proc. R.S.E. V, 1866, and VI, 1868.
58 Physiolog. Notizen (9), p. 425, 1895. Cf. Strasbürger, Ueber die Wirkungssphäre der Kerne und die Zellgrösse, Histolog. Beitr. (5), pp. 95–129, 1893; J. J. Gerassimow, Ueber die Grösse des Zellkernes, Beih. Bot. Centralbl. XVIII, 1905; also G. Levi and T. Terni, Le variazioni dell’ indice plasmatico-nucleare durante l’intercinesi, Arch. Ital. di Anat. X, p. 545, 1911.
59 Arch. f. Entw. Mech. IV, 1898, pp. 75, 247.
60 Conklin, E. G., Cell-size and nuclear-size, J. Exp. Zool. XII. pp. 1–98, 1912.
61 Thus the fibres of the crystalline lens are of the same size in large and small dogs; Rabl, Z. f. w. Z. LXVII, 1899. Cf. (int. al.) Pearson, On the Size of the Blood-corpuscles in Rana, Biometrika, VI, p. 403, 1909. Dr Thomas Young caught sight of the phenomenon, early in last century: “The solid particles of the blood do not by any means vary in magnitude in the same ratio with the bulk of the animal,” Natural Philosophy, ed. 1845, p. 466; and Leeuwenhoek and Stephen Hales were aware of it a hundred years before. But in this case, though the blood-corpuscles show no relation of magnitude to the size of the animal, they do seem to have some relation to its activity. At least the corpuscles in the sluggish Amphibia are much the largest known to us, while the smallest are found among the deer and other agile and speedy mammals. (Cf. Gulliver, P.Z.S. 1875, p. 474, etc.) This apparent correlation may have its bearing on modern views of the surface-condensation or adsorption of oxygen in the blood-corpuscles, a process which would be greatly facilitated and intensified by the increase of surface due to their minuteness.
62 Cf. P. Enriques, La forma come funzione della grandezza: Ricerche sui gangli nervosi degli Invertebrati, Arch. f. Entw. Mech. XXV, p. 655, 1907–8.
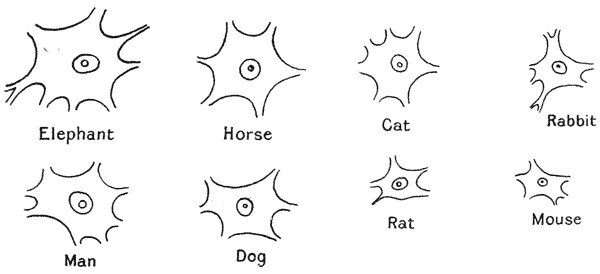
Fig. 1. Motor ganglion-cells, from the cervical spinal cord.
(From Minot, after Irving Hardesty.)
In Fig. 1 we have certain identical nerve-cells taken from various mammals, from the mouse to the elephant, all represented on the same scale of magnification; and we see at once that they are all of much the same order of magnitude. The nerve-cell of the elephant is about twice that of the mouse in linear dimensions, and therefore about eight times greater in volume, or mass. But making some allowance for difference of shape, the linear dimensions of the elephant are to those of the mouse in a ratio certainly not less than one to fifty; from which it would follow that the bulk of the larger animal is something like 125,000 times that of the less. And it also follows, the size of the nerve-cells being {37} about as eight to one, that, in corresponding parts of the nervous system of the two animals, there are more than 15,000 times as many individual cells in one as in the other. In short we may (with Enriques) lay it down as a general law that among animals, whether large or small, the ganglion-cells vary in size within narrow limits; and that, amidst all the great variety of structural type of ganglion observed in different classes of animals, it is always found that the smaller species have simpler ganglia than the larger, that is to say ganglia containing a smaller number of cellular elements63. The bearing of such simple facts as this upon the cell-theory in general is not to be disregarded; and the warning is especially clear against exaggerated attempts to correlate physiological processes with the visible mechanism of associated cells, rather than with the system of energies, or the field of force, which is associated with them. For the life of {38} the body is more than the sum of the properties of the cells of which it is composed: as Goethe said, “Das Lebendige ist zwar in Elemente zerlegt, aber man kann es aus diesen nicht wieder zusammenstellen und beleben.”
Among certain lower and microscopic organisms, such for instance as the Rotifera, we are still more palpably struck by the small number of cells which go to constitute a usually complex organ, such as kidney, stomach, ovary, etc. We can sometimes number them in a few units, in place of the thousands that make up such an organ in larger, if not always higher, animals. These facts constitute one among many arguments which combine to teach us that, however important and advantageous the subdivision of organisms into cells may be from the constructional, or from the dynamical point of view, the phenomenon has less essential importance in theoretical biology than was once, and is often still, assigned to it.
Again, just as Sachs shewed that there was a limit to the amount of cytoplasm which could gather round a single nucleus, so Boveri has demonstrated that the nucleus itself has definite limitations of size, and that, in cell-division after fertilisation, each new nucleus has the same size as its parent-nucleus64.
In all these cases, then, there are reasons, partly no doubt physiological, but in very large part purely physical, which set limits to the normal magnitude of the organism or of the cell. But as we have already discussed the existence of absolute and definite limitations, of a physical kind, to the possible increase in magnitude of an organism, let us now enquire whether there be not also a lower limit, below which the very existence of an organism is impossible, or at least where, under changed conditions, its very nature must be profoundly modified.
Among the smallest of known organisms we have, for instance, Micromonas mesnili, Bonel, a flagellate infusorian, which measures about ·34 µ, or ·00034 mm., by ·00025 mm.; smaller even than this we have a pathogenic micrococcus of the rabbit, M. progrediens, Schröter, the diameter of which is said to be only ·00015 mm. or ·15 µ, or 1·5 × 10−5 cm.,—about equal to the thickness of {39} the thinnest gold-leaf; and as small if not smaller still are a few bacteria and their spores. But here we have reached, or all but reached the utmost limits of ordinary microscopic vision; and there remain still smaller organisms, the so-called “filter-passers,” which the ultra-microscope reveals, but which are mainly brought within our ken only by the maladies, such as hydrophobia, foot-and-mouth disease, or the “mosaic” disease of the tobacco-plant, to which these invisible micro-organisms give rise65. Accordingly, since it is only by the diseases which they occasion that these tiny bodies are made known to us, we might be tempted to suppose that innumerable other invisible organisms, smaller and yet smaller, exist unseen and unrecognised by man.
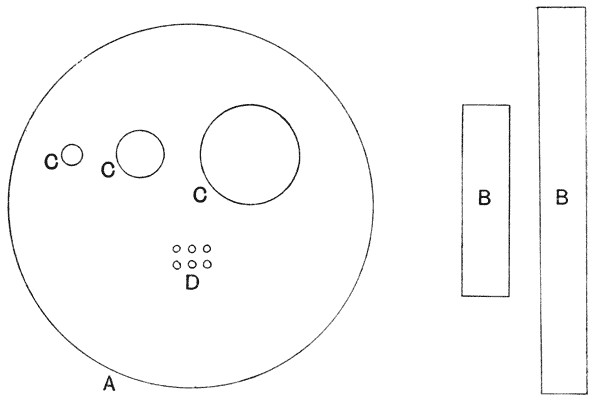
Fig. 2. Relative magnitudes of: A, human blood-corpuscle (7·5 µ in diameter); B, Bacillus anthracis (4 – 15 µ × 1 µ); C, various Micrococci (diam. 0·5 – 1 µ, rarely 2 µ); D, Micromonas progrediens, Schröter (diam. 0·15 µ).
To illustrate some of these small magnitudes I have adapted the preceding diagram from one given by Zsigmondy66. Upon the {40} same scale the minute ultramicroscopic particles of colloid gold would be represented by the finest dots which we could make visible to the naked eye upon the paper.
A bacillus of ordinary, typical size is, say, 1 µ in length. The length (or height) of a man is about a million and three-quarter times as great, i.e. 1·75 metres, or 1·75 × 106 µ; and the mass of the man is in the neighbourhood of five million, million, million (5 × 1018) times greater than that of the bacillus. If we ask whether there may not exist organisms as much less than the bacillus as the bacillus is less than the dimensions of a man, it is very easy to see that this is quite impossible, for we are rapidly approaching a point where the question of molecular dimensions, and of the ultimate divisibility of matter, begins to call for our attention, and to obtrude itself as a crucial factor in the case.
Clerk Maxwell dealt with this matter in his article “Atom67,” and, in somewhat greater detail, Errera discusses the question on the following lines68. The weight of a hydrogen molecule is, according to the physical chemists, somewhere about 8·6 × 2 × 10−22 milligrammes; and that of any other element, whose molecular weight is M, is given by the equation
(M) = 8·6 × M × 10−22 .
Accordingly, the weight of the atom of sulphur may be taken as
8·6 × 32 × 10−22 mgm. = 275 × 10−22 mgm.
The analysis of ordinary bacteria shews them to consist69 of about 85% of water, and 15% of solids; while the solid residue of vegetable protoplasm contains about one part in a thousand of sulphur. We may assume, therefore, that the living protoplasm contains about
1⁄1000 × 15⁄100 = 15 × 10−5
parts of sulphur, taking the total weight as = 1.
But our little micrococcus, of 0·15 µ in diameter, would, if it were spherical, have a volume of
π⁄6 × 0·153 µ = 18 × 10−4 cubic microns; {41}
and therefore (taking its density as equal to that of water), a weight of
18 × 10−4 × 10−9 = 18 × 10−13 mgm.
But of this total weight, the sulphur represents only
18 × 10−13 × 15 × 10−5 = 27 × 10−17 mgm.
And if we divide this by the weight of an atom of sulphur, we have
(27 × 10−17) ÷ (275 × 10−22) = 10,000, or thereby.
According to this estimate, then, our little Micrococcus progrediens should contain only about 10,000 atoms of sulphur, an element indispensable to its protoplasmic constitution; and it follows that an organism of one-tenth the diameter of our micrococcus would only contain 10 sulphur-atoms, and therefore only ten chemical “molecules” or units of protoplasm!
It may be open to doubt whether the presence of sulphur be really essential to the constitution of the proteid or “protoplasmic” molecule; but Errera gives us yet another illustration of a similar kind, which is free from this objection or dubiety. The molecule of albumin, as is generally agreed, can scarcely be less than a thousand times the size of that of such an element as sulphur: according to one particular determination70, serum albumin has a constitution corresponding to a molecular weight of 10,166, and even this may be far short of the true complexity of a typical albuminoid molecule. The weight of such a molecule is
8·6 × 10166 × 10−22 = 8·7 × 10−18 mgm.
Now the bacteria contain about 14% of albuminoids, these constituting by far the greater part of the dry residue; and therefore (from equation (5)), the weight of albumin in our micrococcus is about
14⁄100 × 18 × 10−13 = 2·5 × 10−13 mgm.
If we divide this weight by that which we have arrived at as the weight of an albumin molecule, we have
2·5 × 10−13 ÷ (8·7 × 10−18) = 2·9 × 10−4 ,
in other words, our micrococcus apparently contains something less than 30,000 molecules of albumin. {42}
According to the most recent estimates, the weight of the hydrogen molecule is somewhat less than that on which Errera based his calculations, namely about 16 × 10−22 mgms. and according to this value, our micrococcus would contain just about 27,000 albumin molecules. In other words, whichever determination we accept, we see that an organism one-tenth as large as our micrococcus, in linear dimensions, would only contain some thirty molecules of albumin; or, in other words, our micrococcus is only about thirty times as large, in linear dimensions, as a single albumin molecule71.
We must doubtless make large allowances for uncertainty in the assumptions and estimates upon which these calculations are based; and we must also remember that the data with which the physicist provides us in regard to molecular magnitudes are, to a very great extent, maximal values, above which the molecular magnitude (or rather the sphere of the molecule’s range of motion) is not likely to lie: but below which there is a greater element of uncertainty as to its possibly greater minuteness. But nevertheless, when we shall have made all reasonable allowances for uncertainty upon the physical side, it will still be clear that the smallest known bodies which are described as organisms draw nigh towards molecular magnitudes, and we must recognise that the subdivision of the organism cannot proceed to an indefinite extent, and in all probability cannot go very much further than it appears to have done in these already discovered forms. For, even, after giving all due regard to the complexity of our unit (that is to say the albumin-molecule), with all the increased possibilities of interrelation with its neighbours which this complexity implies, we cannot but see that physiologically, and comparatively speaking, we have come down to a very simple thing.
While such considerations as these, based on the chemical composition of the organism, teach us that there must be a definite lower limit to its magnitude, other considerations of a purely physical kind lead us to the same conclusion. For our discussion of the principle of similitude has already taught us that, long before we reach these almost infinitesimal magnitudes, the {43} diminishing organism will have greatly changed in all its physical relations, and must at length arrive under conditions which must surely be incompatible with anything such as we understand by life, at least in its full and ordinary development and manifestation.
We are told, for instance, that the powerful force of surface-tension, or capillarity, begins to act within a range of about 1 ⁄ 500,000 of an inch, or say 0·05 µ. A soap-film, or a film of oil upon water, may be attenuated to far less magnitudes than this; the black spots upon a soap-bubble are known, by various concordant methods of measurement, to be only about 6 × 10−7 cm., or about ·006 µ thick, and Lord Rayleigh and M. Devaux72 have obtained films of oil of ·002 µ, or even ·001 µ in thickness.
But while it is possible for a fluid film to exist in these almost molecular dimensions, it is certain that, long before we reach them, there must arise new conditions of which we have little knowledge and which it is not easy even to imagine.
It would seem that, in an organism of ·1 µ in diameter, or even rather more, there can be no essential distinction between the interior and the surface layers. No hollow vesicle, I take it, can exist of these dimensions, or at least, if it be possible for it to do so, the contained gas or fluid must be under pressures of a formidable kind73, and of which we have no knowledge or experience. Nor, I imagine, can there be any real complexity, or heterogeneity, of its fluid or semi-fluid contents; there can be no vacuoles within such a cell, nor any layers defined within its fluid substance, for something of the nature of a boundary-film is the necessary condition of the existence of such layers. Moreover, the whole organism, provided that it be fluid or semi-fluid, can only be spherical in form. What, then, can we attribute, in the way of properties, to an organism of a size as small as, or smaller than, say ·05 µ? It must, in all probability, be a homogeneous, structureless sphere, composed of a very small number of albuminoid or other molecules. Its vital properties and functions must be extraordinarily limited; its specific outward characters, even if we could see it, must be nil; and its specific properties must be little more than those of an ion-laden corpuscle, enabling it to perform {44} this or that chemical reaction, or to produce this or that pathogenic effect. Even among inorganic, non-living bodies, there must be a certain grade of minuteness at which the ordinary properties become modified. For instance, while under ordinary circumstances crystallisation starts in a solution about a minute solid fragment or crystal of the salt, Ostwald has shewn that we may have particles so minute that they fail to serve as a nucleus for crystallisation,—which is as much as to say that they are too minute to have the form and properties of a “crystal”; and again, in his thin oil-films, Lord Rayleigh has noted the striking change of physical properties which ensues when the film becomes attenuated to something less than one close-packed layer of molecules74.
Thus, as Clerk Maxwell put it, “molecular science sets us face to face with physiological theories. It forbids the physiologist from imagining that structural details of infinitely small dimensions [such as Leibniz assumed, one within another, ad infinitum] can furnish an explanation of the infinite variety which exists in the properties and functions of the most minute organisms.” And for this reason he reprobates, with not undue severity, those advocates of pangenesis and similar theories of heredity, who would place “a whole world of wonders within a body so small and so devoid of visible structure as a germ.” But indeed it scarcely needed Maxwell’s criticism to shew forth the immense physical difficulties of Darwin’s theory of Pangenesis: which, after all, is as old as Democritus, and is no other than that Promethean particulam undique desectam of which we have read, and at which we have smiled, in our Horace.
There are many other ways in which, when we “make a long excursion into space,” we find our ordinary rules of physical behaviour entirely upset. A very familiar case, analysed by Stokes, is that the viscosity of the surrounding medium has a relatively powerful effect upon bodies below a certain size. A droplet of water, a thousandth of an inch (25 µ) in diameter, cannot fall in still air quicker than about an inch and a half per second; and as its size decreases, its resistance varies as the diameter, and not (as with larger bodies) as the surface of the {45} drop. Thus a drop one-tenth of that size (2·5 µ), the size, apparently, of the drops of water in a light cloud, will fall a hundred times slower, or say an inch a minute; and one again a tenth of this diameter (say ·25 µ, or about twice as big, in linear dimensions, as our micrococcus), will scarcely fall an inch in two hours. By reason of this principle, not only do the smaller bacteria fall very slowly through the air, but all minute bodies meet with great proportionate resistance to their movements in a fluid. Even such comparatively large organisms as the diatoms and the foraminifera, laden though they are with a heavy shell of flint or lime, seem to be poised in the water of the ocean, and fall in it with exceeding slowness.
The Brownian movement has also to be reckoned with,—that remarkable phenomenon studied nearly a century ago (1827) by Robert Brown, facile princeps botanicorum. It is one more of those fundamental physical phenomena which the biologists have contributed, or helped to contribute, to the science of physics.
The quivering motion, accompanied by rotation, and even by translation, manifested by the fine granular particles issuing from a crushed pollen-grain, and which Robert Brown proved to have no vital significance but to be manifested also by all minute particles whatsoever, organic and inorganic, was for many years unexplained. Nearly fifty years after Brown wrote, it was said to be “due, either directly to some calorical changes continually taking place in the fluid, or to some obscure chemical action between the solid particles and the fluid which is indirectly promoted by heat75.” Very shortly after these last words were written, it was ascribed by Wiener to molecular action, and we now know that it is indeed due to the impact or bombardment of molecules upon a body so small that these impacts do not for the moment, as it were, “average out” to approximate equality on all sides. The movement becomes manifest with particles of somewhere about 20 µ in diameter, it is admirably displayed by particles of about 12 µ in diameter, and becomes more marked the smaller the particles are. The bombardment causes our particles to behave just like molecules of uncommon size, and this {46} behaviour is manifested in several ways76. Firstly, we have the quivering movement of the particles; secondly, their movement backwards and forwards, in short, straight, disjointed paths; thirdly, the particles rotate, and do so the more rapidly the smaller they are, and by theory, confirmed by observation, it is found that particles of 1 µ in diameter rotate on an average through 100° per second, while particles of 13 µ in diameter turn through only 14° per minute. Lastly, the very curious result appears, that in a layer of fluid the particles are not equally distributed, nor do they all ever fall, under the influence of gravity, to the bottom. But just as the molecules of the atmosphere are so distributed, under the influence of gravity, that the density (and therefore the number of molecules per unit volume) falls off in geometrical progression as we ascend to higher and higher layers, so is it with our particles, even within the narrow limits of the little portion of fluid under our microscope. It is only in regard to particles of the simplest form that these phenomena have been theoretically investigated77, and we may take it as certain that more complex particles, such as the twisted body of a Spirillum, would show other and still more complicated manifestations. It is at least clear that, just as the early microscopists in the days before Robert Brown never doubted but that these phenomena were purely vital, so we also may still be apt to confuse, in certain cases, the one phenomenon with the other. We cannot, indeed, without the most careful scrutiny, decide whether the movements of our minutest organisms are intrinsically “vital” (in the sense of being beyond a physical mechanism, or working model) or not. For example, Schaudinn has suggested that the undulating movements of Spirochaete pallida must be due to the presence of a minute, unseen, “undulating membrane”; and Doflein says of the same species that “sie verharrt oft mit eigenthümlich zitternden Bewegungen zu einem Orte.” Both movements, the trembling or quivering {47} movement described by Doflein, and the undulating or rotating movement described by Schaudinn, are just such as may be easily and naturally interpreted as part and parcel of the Brownian phenomenon.
While the Brownian movement may thus simulate in a deceptive way the active movements of an organism, the reverse statement also to a certain extent holds good. One sometimes lies awake of a summer’s morning watching the flies as they dance under the ceiling. It is a very remarkable dance. The dancers do not whirl or gyrate, either in company or alone; but they advance and retire; they seem to jostle and rebound; between the rebounds they dart hither or thither in short straight snatches of hurried flight; and turn again sharply in a new rebound at the end of each little rush. Their motions are wholly “erratic,” independent of one another, and devoid of common purpose. This is nothing else than a vastly magnified picture, or simulacrum, of the Brownian movement; the parallel between the two cases lies in their complete irregularity, but this in itself implies a close resemblance. One might see the same thing in a crowded market-place, always provided that the bustling crowd had no business whatsoever. In like manner Lucretius, and Epicurus before him, watched the dust-motes quivering in the beam, and saw in them a mimic representation, rei simulacrum et imago, of the eternal motions of the atoms. Again the same phenomenon may be witnessed under the microscope, in a drop of water swarming with Paramoecia or suchlike Infusoria; and here the analogy has been put to a numerical test. Following with a pencil the track of each little swimmer, and dotting its place every few seconds (to the beat of a metronome), Karl Przibram found that the mean successive distances from a common base-line obeyed with great exactitude the “Einstein formula,” that is to say the particular form of the “law of chance” which is applicable to the case of the Brownian movement78. The phenomenon is (of course) merely analogous, and by no means identical with the Brownian movement; for the range of motion of the little active organisms, whether they be gnats or infusoria, is vastly greater than that of the minute particles which are {48} passive under bombardment; but nevertheless Przibram is inclined to think that even his comparatively large infusoria are small enough for the molecular bombardment to be a stimulus, though not the actual cause, of their irregular and interrupted movements.
There is yet another very remarkable phenomenon which may come into play in the case of the minutest of organisms; and this is their relation to the rays of light, as Arrhenius has told us. On the waves of a beam of light, a very minute particle (in vacuo) should be actually caught up, and carried along with an immense velocity; and this “radiant pressure” exercises its most powerful influence on bodies which (if they be of spherical form) are just about ·00016 mm., or ·16 µ in diameter. This is just about the size, as we have seen, of some of our smallest known protozoa and bacteria, while we have some reason to believe that others yet unseen, and perhaps the spores of many, are smaller still. Now we have seen that such minute particles fall with extreme slowness in air, even at ordinary atmospheric pressures: our organism measuring ·16 µ would fall but 83 metres in a year, which is as much as to say that its weight offers practically no impediment to its transference, by the slightest current, to the very highest regions of the atmosphere. Beyond the atmosphere, however, it cannot go, until some new force enable it to resist the attraction of terrestrial gravity, which the viscosity of an atmosphere is no longer at hand to oppose. But it is conceivable that our particle may go yet farther, and actually break loose from the bonds of earth. For in the upper regions of the atmosphere, say fifty miles high, it will come in contact with the rays and flashes of the Northern Lights, which consist (as Arrhenius maintains) of a fine dust, or cloud of vapour-drops, laden with a charge of negative electricity, and projected outwards from the sun. As soon as our particle acquires a charge of negative electricity it will begin to be repelled by the similarly laden auroral particles, and the amount of charge necessary to enable a particle of given size (such as our little monad of ·16 µ) to resist the attraction of gravity may be calculated, and is found to be such as the actual conditions can easily supply. Finally, when once set free from the entanglement of the earth’s {49} atmosphere, the particle may be propelled by the “radiant pressure” of light, with a velocity which will carry it.—like Uriel gliding on a sunbeam,—as far as the orbit of Mars in twenty days, of Jupiter in eighty days, and as far as the nearest fixed star in three thousand years! This, and much more, is Arrhenius’s contribution towards the acceptance of Lord Kelvin’s hypothesis that life may be, and may have been, disseminated across the bounds of space, throughout the solar system and the whole universe!
It may well be that we need attach no great practical importance to this bold conception; for even though stellar space be shewn to be mare liberum to minute material travellers, we may be sure that those which reach a stellar or even a planetary bourne are infinitely, or all but infinitely, few. But whether or no, the remote possibilities of the case serve to illustrate in a very vivid way the profound differences of physical property and potentiality which are associated in the scale of magnitude with simple differences of degree.
Fig. 1. Motor ganglion-cells, from the cervical spinal cord.
(From Minot, after Irving Hardesty.)
63 While the difference in cell-volume is vastly less than that between the volumes, and very much less also than that between the surfaces, of the respective animals, yet there is a certain difference; and this it has been attempted to correlate with the need for each cell in the many-celled ganglion of the larger animal to possess a more complex “exchange-system” of branches, for intercommunication with its more numerous neighbours. Another explanation is based on the fact that, while such cells as continue to divide throughout life tend to uniformity of size in all mammals, those which do not do so, and in particular the ganglion cells, continue to grow, and their size becomes, therefore, a function of the duration of life. Cf. G. Levi, Studii sulla grandezza delle cellule, Arch. Ital. di Anat. e di Embryolog. V, p. 291, 1906.
64 Boveri. Zellen-studien, V. Ueber die Abhängigkeit der Kerngrösse und Zellenzahl der Seeigellarven von der Chromosomenzahl der Ausgangszellen. Jena, 1905.
65 Recent important researches suggest that such ultra-minute “filter-passers” are the true cause of certain acute maladies commonly ascribed to the presence of much larger organisms; cf. Hort, Lakin and Benians, The true infective Agent in Cerebrospinal Fever, etc., J. Roy. Army Med. Corps, Feb. 1910.
66 Zur Erkenntniss der Kolloide, 1905, p. 122; where there will be found an interesting discussion of various molecular and other minute magnitudes.
67 Encyclopaedia Britannica, 9th edit., vol. III, p. 42, 1875.
68 Sur la limite de petitesse des organismes, Bull. Soc. R. des Sc. méd. et nat. de Bruxelles, Jan. 1903; Rec. d’œuvres (Physiol. générale), p. 325.
69 Cf. A. Fischer, Vorlesungen über Bakterien, 1897, p. 50.
70 F. Hofmeister, quoted in Cohnheim’s Chemie der Eiweisskörper, 1900, p. 18.
71 McKendrick arrived at a still lower estimate, of about 1250 proteid molecules in the minutest organisms. Brit. Ass. Rep. 1901, p. 808.
72 Cf. Perrin, Les Atomes, 1914, p. 74.
73 Cf. Tait, On Compression of Air in small Bubbles, Proc. R. S. E. V, 1865.
74 Phil. Mag. XLVIII, 1899; Collected Papers, IV, p. 430.
75 Carpenter, The Microscope, edit. 1862, p. 185.
76 The modern literature on the Brownian Movement is very large, owing to the value which the phenomenon is shewn to have in determining the size of the atom. For a fuller, but still elementary account, see J. Cox, Beyond the Atom, 1913, pp. 118–128; and see, further, Perrin, Les Atomes, pp. 119–189.
77 Cf. R. Gans, Wie fallen Stäbe und Scheiben in einer reibenden Flüssigkeit? Münchener Bericht, 1911, p. 191; K. Przibram, Ueber die Brown’sche Bewegung nicht kugelförmiger Teilchen, Wiener Ber. 1912, p. 2339.
78 Ueber die ungeordnete Bewegung niederer Thiere, Pflüger’s Archiv, CLIII, p. 401, 1913.
