автордың кітабын онлайн тегін оқу A Practical Treatise on the Manufacture of Perfumery
A PRACTICAL TREATISE
ON THE
MANUFACTURE OF PERFUMERY:
COMPRISING
DIRECTIONS FOR MAKING ALL KINDS OF PERFUMES, SACHET
POWDERS, FUMIGATING MATERIALS, DENTIFRICES,
COSMETICS, ETC., ETC.,
WITH A FULL ACCOUNT OF THE
VOLATILE OILS, BALSAMS, RESINS, AND OTHER NATURAL
AND ARTIFICIAL PERFUME-SUBSTANCES, INCLUDING
THE MANUFACTURE OF FRUIT ETHERS, AND
TESTS OF THEIR PURITY.
BY
Dr. C. DEITE,
Assisted by L. BORCHERT, F. EICHBAUM, E. KUGLER,
H. TOEFFNER, and other experts.
FROM THE GERMAN BY
WILLIAM T. BRANNT,
EDITOR OF "THE TECHNO-CHEMICAL RECEIPT-BOOK."
ILLUSTRATED BY TWENTY-EIGHT ENGRAVINGS.
PHILADELPHIA:
HENRY CAREY BAIRD & CO.,
INDUSTRIAL PUBLISHERS, BOOKSELLERS AND IMPORTERS,
810 WALNUT STREET.
1892.
Copyright by
HENRY CAREY BAIRD & CO.
1892.
Printed at the COLLINS PRINTING HOUSE,
705 Jayne Street,
Philadelphia, U. S. A.
PREFACE.
A translation of the portion of the "Handbuch der Parfümerie-und Toiletteseifenfabrikation," edited by Dr. C. Deite, relating to perfumery and cosmetics, is presented to the English reading public with the full confidence that it will not only fill a useful place in technical literature, but will also prove—for what it is chiefly intended—a ready book of reference and a practical help and guide for the perfumer's laboratory. The names of the editor and his co-workers are a sufficient guaranty of its value and practical usefulness, they all being experienced men, well schooled each in the particular branch of the industry, the treatment of which has been assigned to him.
The most suitable and approved formulæ, tested by experience, have been given; and special attention has been paid to the description of the raw materials, as well as to the various methods of testing them, the latter being of special importance, since in no other industry has the manufacturer to contend with such gross and universal adulteration of raw materials.
It is hoped that the additions made here and there by the translator, as well as the portion relating to the manufacture of "Fruit Ethers," added by him, may contribute to the interest and usefulness of the treatise.
Finally, it remains only to be stated that, with their usual liberality, the publishers have spared no expense in the proper illustration and the mechanical production of the book; and, as is their universal practice, have caused it to be provided with a copious table of contents and a very full index, which will add additional value by rendering any subject in it easy and prompt of reference.
W. T. B.
Philadelphia, May 2, 1892.
CONTENTS.
CHAPTER I.
HISTORICAL NOTICE OF PERFUMERY.
PAGEConsumption of perfume-substances by the early nations of the Orient
17Perfume-substances as an offering to the gods and their use for embalming the dead; Arts of the toilet in ancient times
18Perfume-substances used by the Hebrews; Olibanum and the mode of gaining it in ancient times, as described by Herodotus
19Pliny's account of olibanum
20Practice of anointing the entire body customary among the ancients; The holy oil prescribed by Moses; Origin of the sweet-scented ointment "myron"
21Luxurious use of ointments in Athens, and the special ointments used for each part of the body; Introduction of ointments in Rome, and edict prohibiting the sale of foreign ointments; Plutarch on the extravagant use of ointments in Rome
22Ancient books containing directions for preparing ointments; Directions for rose ointment, according to Dioscorides
23Ancient process of distilling volatile oils; Dioscorides's directions for making animal fats suitable for the reception of perfumes; Consumption of perfume-substances by the ancient Romans; Condition of the ancient ointment-makers
24Use of red and white paints, hair-dyes, and depilatories by the Romans
25Peculiar substance for cleansing the teeth used by the Roman ladies; Perfumeries and cosmetics in the Middle Ages; Receipts for cosmetics in the writings of Arabian physicians, and of Guy de Chanlios
26Giovanni Marinello's work on "Cosmetics for Ladies;" Introduction of the arts of the toilet into France, by Catherine de Medici and Margaret of Valois
27Extravagant use of cosmetics in France from the commencement of the seventeenth to the middle of the eighteenth century
28Importance of the perfumer's craft in France; Chief seats of the French perfumery industry
29Privileges of the
parfumeurs-gantiersin France; Use of perfumes in England; Act of Parliament prohibiting the use of perfumeries, false hair, etc., for deceiving a man and inveigling him into matrimony
30 CHAPTER II.
THE PERFUME-MATERIALS FOR THE MANUFACTURE OF PERFUMERY.
Derivation of the perfume-substances; Animal substances used; Occurrence of volatile oils in plants
31Families of plants richest in oil; Central Europe the actual flower garden of the perfumer; Principal localities for the cultivation of plants
32Volatile oils and their properties
33Principal divisions of volatile oils
34Constitution of terpenes; Concentrated volatile oils
35Modes of gaining volatile oils; Expression
36Clarification of the oil
37Filter for clarifying the oil, illustrated and described
38Distillation
39Apparatus for determining the percentage of volatile oil a vegetable substance will yield, illustrated and described
40Various stills for the distillation of volatile oils, illustrated and described
41Distillation of volatile oils by means of hot air; Separation of the oil and water; Florentine flasks, illustrated and described
46Separator-funnel, illustrated and described
47Extraction
48Various apparatuses for extraction, illustrated and described
49Heyl's distilling apparatus
57Maceration or infusion; Pomades; Purification of the fats used in the maceration process
58 Huiles antiques; Old French process of maceration; Piver's maceration apparatus, illustrated and described
59Flowers for which maceration is employed; Absorption or
enfleurage 60Apparatuses for absorption, illustrated and described
61Flowers for which the absorption process is employed; Storage of volatile oils
65 CHAPTER III.
TESTING VOLATILE OILS.
Extensive adulteration of volatile oils; Testing volatile oils as to odor and taste
66Recognition of an adulteration with fat oil
67Detection of alcohol or spirit of wine; Dragendorff's test
68Hager's tannin test
69Detection of chloroform; Detection of benzine
71Quantitative determination of adulterations with alcohol, chloroform, and benzine
72Detection of adulterations with terpenes or terpene-like fluids
73Detection of adulterations with volatile oils of a lower quality; Test with iodine
74Hoppe's nitroprusside of copper test
75Table showing the behavior of volatile oils free from oxygen towards nitroprusside of copper
76Hager's alcohol and sulphuric acid test; Hager's guaiacum reaction
78Division of the volatile oils with reference to the guaiacum reaction
79Hübl's iodine method
80A. Kremel's test by titration or saponification with alcoholic potash lye
81Utilization of Maumené's test by F. R. Williams
82Planchon's proposed procedure for the recognition of a volatile oil
83 CHAPTER IV.
THE VOLATILE OILS USED IN PERFUMERY.
Acacia oil or oil of cassie; Almond oil (bitter)
87Adulterations of oil of bitter almonds and their detection
90Angelica oil
92Anise-seed oil
93Star anise oil
94Balm oil; Basil oil; Bayberry oil, or oil of bay leaves
96Bergamot oil; Testing bergamot oil as to its purity
97Cajeput oil
98Camomile or chamomile oil; Blue camomile oil; Green camomile oil
99Caraway oil; Recognition of the purity of caraway oil
100Cedar oil; Cherry-laurel oil
101Detection of oil of mirbane in cherry-laurel oil; Cinnamon oils; Ceylon cinnamon oil
102Cassia oil
103Cinnamon-root oil and oil of cinnamon leaves; Quantitative determination of cinnamaldehyde in cassia oil
104Detection of adulterations in cassia oil; Citron oil
106Detection of adulterations in citron oil; Citronella oil; Detection of adulterations in citronella oil
107Oil of cloves
108Test for the value of oil of cloves
109Eucalyptus oil
110Fennel oil
111Geranium oil, palmarosa oil, Turkish geranium oil; East Indian geranium oil; French and African geranium oils
112Adulterations of geranium oils; Jasmine oil, or oil of jessamine
113Juniper oil
114Lavender oil; Spike oil
115Detection of adulterations of lavender oil; Lemon oil; Sponge process of obtaining lemon oil
116Écuelle process
117Distillation; Apparatus combining the écuelle and distilling processes, illustrated and described
118Adulterations of oil of lemons and their detection: Lilac oil; Oil of limes
121Licari oil, linaloë oil; Marjoram oils; Spanish marjoram oil
122Mignonette oil; Myrrh oil
123Nutmeg oils; Mace oil; Adulterations of mace oil and their detection
124Opopanax oil; Orange-peel oil, Portugal oil or essence of Portugal; Mandarin oil
125Orange-flower oil or neroli oil; Neroli Portugal oil; Cultivation of the orange on the French Riviera and yield of orange blossoms; Characteristics of oil of orange flowers
126Adulterations of neroli oil and their detection
127Petit-grain oil; Oil of orris root
129Patchouli oil
130Varieties and characteristics of patchouli oil
131Peppermint oil; Oil of curled mint; Peppermint oil and its varieties
132American oils of peppermint of high reputation; Mode of distinguishing American, German, and English oils of peppermint
133Adulterants of peppermint oil and their detection
134Poley oil
135Pimento oil or oil of allspice; Rose oil or attar of roses; Principal localities of its production; Schimmel & Co.'s, of Leipzic, Germany, experiment to obtain oil from indigenous roses
136The rose-oil industry in Bulgaria; Methods of gathering and distilling the roses
137Characteristics of pure rose oil
138Manner of judging the genuineness of rose oil; Process for the insulation and determination of stearoptene in rose oil
139Adulteration of rose oil with ginger-grass oil
140Test for the adulteration of rose oil with ginger-grass oil employed in Bulgaria
141Adulterants of rose oil
142Tests for rose oil; Approximate quantitative determination of spermaceti in rose oil
143Rosemary oil; Detection of adulterations in rosemary oil
144Rosewood oil or rhodium oil; Sandal-wood oil; Sassafras oil; Characteristics of sassafras oil
145Thyme oil
147Oil of turpentine; Austrian oil of turpentine; German oil of turpentine; French oil of turpentine; Venetian oil of turpentine
148American oil of turpentine; Pine oil; Dwarf pine oil; Krummholz or Latschenoel; Pine-leaf oil; Templin oil (Kienoel); Balsam-pine oil
149Oil of verbena; Oil of violet; Vitivert or vetiver oil
150Wintergreen oil
151Birch oil; Artificial preparation of methyl salicylate
152Adulteration of wintergreen oil and its detection; Ylang-ylang oil
153Cananga oil
154 CHAPTER V.
RESINS AND BALSAMS.
Elementary constituents of resins; Division of resins; Hard resins; Soft resins or balsams; Gum-resins
155Diffusion of resins in the vegetable kingdom; Benzoin
156Varieties of benzoin and their characteristics
157Peru balsam and mode of obtaining it
159White Peru balsam
160Characteristics of Peru balsam
161Adulterants of Peru balsam and their detection
162Tolu balsam and its characteristics
166A new variety of Tolu balsam
167Storax; Liquid storax and its characteristics
168Adulteration of liquid storax and its detection
170Storax in grains; Ordinary storax
171American storax, white Peru balsam, white Indian balsam, or liquid-ambar; Myrrh
172Myrrha electa and its characteristics
173Constitution of myrrh
174Adulteration of myrrh and its detection
175Opopanax; Olibanum or frankincense
176Commercial varieties of olibanum; Sandarac and its characteristics
177 CHAPTER VI.
PERFUME-SUBSTANCES FROM THE ANIMAL KINGDOM.
Musk and its varieties; Musk sacs, illustrated and described
178Characteristics of Tonkin musk
180Musk of the American musk-rat as a substitute for genuine musk
181Other possible substitutes for the musk-deer; Artificial musk
182Adulterations of musk and their detection
183Civet
184Castor and its varieties
185Adulterations of castor; Ambergris
186Constituents of ambergris
187Adulterations of ambergris
188 CHAPTER VII.
ARTIFICIAL PERFUME-MATERIALS.
Conversion of oil of turpentine into oil of lemons by Bouchardat
and Lafont
189Cumarin, its occurrence and properties
190Varieties of tonka beans found in commerce
191Preparation of cumarin from tonka beans; Artificial preparation of cumarin from salicylic acid
192Synthetical preparation of cumarin; Heliotropin or piperonal and its characteristics
193Preparation of heliotropin
194Vanillin; Characteristics of the vanilla
195Artificial preparation of vanillin
196Characteristics of vanillin
197Adulteration of vanillin, and its detection; Nitrobenzol
198Characteristics of nitrobenzol or oil of mirbane; adulteration of nitrobenzol and its detection
199Fruit ethers and their characteristics
200Acetic amyl ether or amyl acetate, its preparation and use; Acetic ether or ethyl acetate and its preparation
201Benzoic ether or ethyl benzoate and its preparation
204Butyric ethyl ether or ethyl butyrate; Preparation of butyric acid
205Preparation of butyric ether
207St. John's bread or carob as material for the preparation of butyric ether
209Formic ethyl ether, or ethyl formate and its preparation
210Nitrous ether or ethyl nitrate and its preparation according to Kopp's method
211Preparation and use of nitrous ether in England and America
212Valerianic amyl ether or amyl valerate and its preparation
214Valerianic ethyl ether; Apple ether; Apricot ether; Cherry ether; Pear ether; Pineapple ether; Strawberry ether; Preparation of fruit essences; Apple essence; Apricot essence
216Cherry essence; Currant essence; Grape essence; Lemon essence; Melon essence; Orange essence; Peach essence; Pear essence; Pineapple essence; Plum essence
217Raspberry essence; Strawberry essence
218 CHAPTER VIII.
ALCOHOLIC PERFUMES.
Division of alcoholic perfumes; What constitutes the art of the perfumer; Qualities of flower-pomades and their designation
219Storage of flower-pomades; Extraction of flower-pomades
220Apparatus for making alcoholic extracts from flower-pomades, illustrated and described
221Beyer frères improved apparatus, illustrated and described
223Tinctures and extracts and their preparation
225Beyer frères apparatus for the preparation of tinctures, illustrated and described
226Musk tincture; Civet tincture
228Ambergris tincture; Castor tincture; Benzoin tincture; Peru balsam tincture; Tolu balsam tincture
229Olibanum tincture; Opopanax tincture; Storax tincture; Myrrh tincture; Musk-seed or abelmosk tincture
230Angelica root tincture; Orris-root tincture; Musk-root or sumbul-root tincture; Tonka-bean tincture
231Cumarin tincture; Heliotropin tincture; Vanilla tincture; Vanillin tincture
232Vitivert tincture; Juniper-berry tincture; Patchouli extract
233Tinctures from volatile oils; Almond-oil (bitter) tincture; Balm-oil tincture; Bergamot-oil tincture; Canango-oil tincture
234Cassia-oil tincture; Cedar-oil tincture; Cinnamon-oil tincture; Citronella-oil tincture; Clove-oil tincture; Eucalyptus-oil tincture; Geranium-oil tincture; Lavender-oil tincture; Lemon-grass-oil tincture; Lemon-oil tincture; Licari-oil tincture; Myrrh-oil tincture; Neroli-oil tincture; Opopanax-oil tincture; Orris-root-oil tincture; Patchouli-oil tincture
235Petit-grain-oil tincture; Pine-leaf-oil tincture; Portugal-oil tincture; Sandal-wood-oil tincture; Verbena-oil tincture; Vitivert-oil tincture; Wintergreen-oil tincture; Ylang-ylang-oil tincture; Rose-oil tincture
236Extraits aux fleurs; Extrait acacia; Extrait cassie; Extrait héliotrope; Extrait jacinthe
237Extrait jasmin; Essence of the odor of linden blossoms; Extrait jonquille; Extrait magnolia; Extrait muguet (lily of the valley); Extrait fleurs de Mai (May flowers)
238Extrait ixora; Extrait orange; Extrait white rose; Extrait rose v. d. centifolie; Extrait violette; Coloring substance for extraits; Extrait de violette de Parme
239Extrait tubereuse; Extrait réséda; Extrait ylang-ylang; Compound odors (bouquets); Extrait Edelweiss; Extrait ess-bouquet
240Extrait spring flower; Extrait bouquet Eugenie; Extrait excelsior; Extrait Frangipani; Extrait jockey club
241Extrait opopanax; Extrait patchouli; Extrait millefleurs; Extrait bouquet Victoria
242Extrait kiss-me-quick; Extrait mogadore; Extrait bouquet Prince Albert; Extrait muse; Extrait new-mown hay; Extrait chypre
243Extrait maréchal; Extrait mousseline; Extraits triple concentrés and their preparations
244Concentrated flower-extract for the preparation of extraits d'Odeurs; Extraits d'Odeurs, quality II
245Extrait violette II; Extrait rose II; Extrait réséda II; Extrait ylang-ylang II
246Extrait new-mown hay II; Extrait chypre II; Extrait ess-bouquet II
247Extrait muguet II; Extrait bouquet Victoria II; Extrait spring flower II; Extrait ixora II
248Extrait Frangipani II; Cologne water (eau de Cologne) and its preparation
249Durability of the volatile oils used in the preparation of Cologne water
250Cologne water, quality I
252Cologne water, quality II; Cologne water, quality III; Cologne water, quality IV; Cologne water, quality V
253Maiglöckchen eau de Cologne; Various other receipts for Cologne water
254Eau de Lavande; Eau de vie de Lavande double ambrée; Eau de Lavande double; Aqua mellis; Eau de Lisbonne
255 CHAPTER IX.
DRY PERFUMES.
Use of dry perfumes in ancient times; Sachet powders and their preparation
256Sachet à la rose; Sachet à la violette; Hliotrope sachet powder; Ylang-ylang sachet powder; Jockey club sachet
257Sachet aux millefleurs; Lily of the valley sachet powder; Patchouli sachet powder; Frangipani sachet powder; Victoria sachet powder; Réséda sachet powder
258Musk sachet powder; Ess-bouquet sachet powder; New-mown hay sachet powder; Orange sachet powder; Solid perfumes with paraffine; White rose
259Ess-bouquet; Lavender odor; Eau de Cologne; Smelling salts; Preston salt and "menthol pungent" as prepared by William W. Bartlett; White smelling salt
260 CHAPTER X.
FUMIGATING ESSENCES, PASTILLES, POWDERS, ETC.
Constitution of fumigating agents; Object of fumigating;
Prejudice against fumigating; Mode of fumigating
262Atomizers; Objections to dry fumigating agents
263Fumigating essences and vinegars; Rose-flower fumigating essence; Flower fumigating essence—héliotrope
264Violet-flower fumigating essence; Oriental flower fumigating essence; Pine odor (for atomizing); Juniper odor; fumigating balsam
265Fumigating water; Fumigating vinegar; Fumigating powders; Ordinary fumigating powder
266Rose fumigating powder; Violet fumigating powder; Orange fumigating powder; New-mown hay fumigating powder
267Fumigating paper; Fumigating pastilles
268Ordinary red fumigating pastilles; Ordinary black fumigating pastilles; Musk fumigating pastilles
269Rose fumigating pastilles; Violet fumigating pastilles; Millefleurs fumigating pastilles; Fumigating lacquer
270 CHAPTER XI.
DENTIFRICES, MOUTH-WATERS, ETC.
Selection of materials for and compounding of dentifrices
272Soap as a constituent of dentifrices; Value of thymol for dentifrices; Object of glycerin in dentifrices
273Tooth and mouth waters; Thymol tooth-water; Eau dentifrice Botot; Eau dentifrice Orientale
274Violet mouth-water; Antiseptic gargle; Odontine; Sozodont; Eau de Botot (improved)
275Quinine tooth-water; Dr. Stahl's tooth-tincture; Esprit de menthe; Arnica tooth-tincture; Myrrh tooth-tincture
276Tooth-pastes and tooth-powders; tooth-paste or odontine
277Thymol tooth-paste; Cherry tooth-paste; Non-fermenting cherry tooth-paste; Odontine paste
278Thymol tooth-powder; Poudre dentifrice; Violet tooth-powder
279Dr. Hufeland's tooth-powder; White tooth-powder; Black tooth-powder; Poudre de corail; Camphor tooth-powder; Opiat liquide pour les dents
280Poudre d'Algérine
281Dr. Hufeland's tooth-soap
282Tooth-soap; Saponaceous tooth-wash
283 CHAPTER XII.
HAIR POMADES, HAIR OILS, AND HAIR TONICS; HAIR DYES AND DEPILATORIES.
Fats used for the preparation of pomades; Reputation of some fats as hair pomades
284Pomades and their preparation; Purification of the fat
285Substances used for coloring pomades; Fine French pomades (flower-pomades); Maceration or extraction of the flowers
286Receipts for some flower-pomades; Pommade à la rose; Pommade à l'acacia; Pommade à la fleur d'orange; Pommade à l'héliotrope
287Pomades according to the German method and their preparation; Foundations for white pomades
288Apple pomade; Bear's grease pomade; Quinine pomades
289Quinine pomades (imitation); Benzoin pomade; Densdorf pomade; Ice pomades; Family pomades
290Strawberry pomade; Fine hair pomade; Pomade for promoting the growth of the hair; Héliotrope pomades
291Jasmine pomade; Emperor pomade; Macassar pomade; Portugal pomade; Herb pomade; Lanolin pomade
292Oriental pomade; Paraffin ice pomade; Neroli pomade; Cheap pomade (red, yellow, white); Mignonette pomade; Castor oil pomades; Princess pomade
293Fine pomade; Beef-marrow pomade; Rogers's pomade for producing a beard; Rose pomade; Fine rose pomade; Finest rose pomade; Salicylic pomade; Victoria pomade; Tonka pomade
294Fine vanilla pomade; Vanilla pomade; Violet pomade; Walnut pomade; Vaseline pomades
295Foundations for vaseline pomades; Bouquet vaseline pomade; Family vaseline pomade; Lily of the valley vaseline pomade; Neroli vaseline pomade
296Mignonette vaseline pomade; Portugal vaseline pomade; Rose vaseline pomades; Fine vaseline pomade (yellow); Vaseline pomade (red); Vaseline pomade (white); Virginia vaseline pomade; Victoria vaseline pomade
297Extra fine vaseline pomade; Stick pomades; Foundations for stick pomades; Manufacture of stick pomades
298Rose-wax pomade; Black-wax pomade; Blonde-wax pomade; Brown-wax pomade
299Cheap wax pomades; Resin pomades; Hair oils; Huiles antiques; Vaseline oil for hair oils; Treatment of oils with benzoin
300Preparation of huiles antiques; Huile antique à la rose; Huile antique au jasmin; Alpine herb oil; Flower hair oil; Peruvian bark hair oil
301Peru hair oil; Burdock root hair oils; Macassar hair oils; Neroli hair oil; Mignonette hair oils; Fine hair oil
302Cheap hair oil (red or yellow); Portugal hair oil; Jasmine hair oil; Vaseline hair oils; Vanilla hair oil; Ylang-ylang hair oil; Philocome hair oil
303Sultana hair oil; Rose hair oil; Tonka hair oil; Violet hair oil; Victoria hair oil; Cheap hair oils; Bandolines and their preparation
304Rose bandoline; Almond bandoline; Brilliantine
305Flower brilliantine No. 1; Brilliantine No. 2
306Brilliantine No. 3; Various formulas for brilliantine
307Hair tonics; Eau Athénienne; Florida water
308Eau de Cologne hair tonic; Eau de quinine
309Eau de quinine (imitation); Honey water; Glycerin hair tonic; Eau lustral (hair restorative); Tea hair tonic
310Locock's lotion for the hair; Shampoo lotion; Shampoo liquid
311Dandruff cures; Dandruff lotion; Bay rum
312Directions for preparing bay rum
313Hair dyes; Requirements of a good hair dye; Gradual darkening of the hair; Use of dilute acids for making the hair lighter
314Use of lead salts, nitrate of silver, and copper salts for dyeing the hair
315Iron salts for dying the hair; Rastikopetra, a Turkish hair dye; Use of potassium permanganate and pyrogallic acid for dyeing the hair
316Kohol, an Egyptian hair dye; The use of henna as a hair dye; Process of coloring hair, dyed red with henna, black
317Use of the juice of green walnut shells for coloring the hair; Bleaching the hair with peroxide of hydrogen; Formulæ for hair dyes
318Single hair dyes; Teinture Orientale (Karsi); Teinture Chinoise (Kohol)
319Potassium permanganate hair dye; Bismuth hair dye; Walnut hair dye; Pyrogallic hair stain
320Double hair dyes; For dyeing brown; For dyeing black; Tannin hair dye
321Melanogène; Eau d'Afrique; Krinochrom; Copper hair dye; Depilatories; Rhusma
322Boettger's depilatory; Bartholow's depilatory
323 CHAPTER XIII.
COSMETICS.
Skin cosmetics; Toilet vinegars; Vinaigre de Bully; Vinaigre de toilette à la rose; Vinaigre de toilette à la violette
324Vinaigre de toilette héliotrope; Vinaigre de toilette orange; Vinaigre de toilette; Aromatic vinegar; English aromatic vinegar
325Toilet vinegar; Washes; Virginal milk (Lait virginal); Rose milk (Lait de rose)
326Almond milk (Lait d'amandes amères)
327Lily milk (Lait de lys); Perfumed glycerin with rose odor; Perfumed glycerin with fruit odor; Perfumed meals and pastes; Farin de noisette (nut meal)
328Farin d'amandes amères (almond meal); Pate d'amandes au miel (honey almond paste); Poudre de riz à la rose
329Poudre de riz héliotrope; Poudre de riz orange; Poudre de riz muguet
330Poudre de riz ixora; Poudre de riz bouquet; Cold creams and lip salves; Cold cream; Vaseline cold cream
331Glycerin cream; Crême de concombre; Glycerin gelée; Glycerin jelly
332Cream of roses; Boroglycerin cream; Récamier cream; Preparations for chapped hands
333Wash for the hands; Nail powder; Lip-salves
334Paints; Pulverulent paints (powders); "Blanc fard" or "Blanc français"
335Mixtures for powders; Coloring substances for powders; Powder for coloring intensely red; Solid paints; Ordinary red paint (rouge)
336Fine red paint (rouge); White paint; Preparation of paints
337Red stick-paint (stick rouge); Moulding the rouge into sticks
339White stick-paint; Rouge en feuilles; Liquid paints; Liquid rouge
340White liquid paint; Fat paints
341Crême de Lys; Crême de rose
342 Index 343A PRACTICAL TREATISE
ON THE
MANUFACTURE OF PERFUMERY.
Since fragrant odors were agreeable to human beings, it was believed that they must be welcome also to the gods, and, to honor them, perfume substances were burned upon the altars. Besides, as an offering to the gods, perfume substances were extensively used by many nations, especially by the Egyptians, for embalming the dead, the process employed by the latter having been transmitted to us by the ancient authors Herodotus and Diodorus.
Furthermore, a desire for ornamentation and to give to the face and body as pleasing an appearance as possible, is common to all mankind. To be sure, the ideas of what constitutes beauty in this respect have varied at different times and among the various nations. But, independent of the savage races, who consider painting and tattooing the body and face an embellishment, and taking into consideration only the earliest civilized nations, it is astonishing how many arts of the toilet have been preserved from the most ancient historical times up to the present. "In the most ancient historical times, people perfumed and painted, frizzed, curled, and dyed the hair as at present, and, in fact, the same cosmetics, only slightly augmented, which were in use hundreds, nay, thousands, of years ago are still employed to-day."[1] It is especially woman, who everywhere exercises the arts of the toilet, while, with the exception of perfumes and agents for the hair, man is but seldom referred to as making use of cosmetics. The young girls of ancient Egypt used red and white paints, colored their pale lips, and anointed their hair with sweet-scented oils; they dyed their eyelashes and eyelids black to impart a brighter lustre to the glance of the eye, and the mother of the wife of the first king of Egypt is said to have already composed a receipt for a hair-dye.
In ancient times olibanum was, without doubt, the most important perfume-substance. It was introduced into commerce by the Phoenicians, and, like many other substances, it received from them its name, which was adopted by other nations. Thus, the Hebrews called the tree lebonah, the Arabs, lubah, while the Greeks named it, λιβανός and the resin derived from it, the celebrated frankincense of the ancients, λιβανωτόςτς, Latin, olibanum. Regarding the mode of gaining the olibanum, some curious ideas prevailed in ancient times. Thus, Herodotus writes: "Arabia is the only country in which olibanum grows, as well as myrrh, cassia, cinnamon and lederum. With the exception of myrrh, the Arabs encounter many difficulties in procuring these products. Olibanum they obtain by burning styrax, for every olibanum tree is guarded by a number of small-sized winged serpents of a variegated appearance, which can be driven away by nothing but styrax vapors." According to Pliny, who gives a very full account of olibanum, Arabia felix received its by-name from the abundance of olibanum and myrrh found there. He states that olibanum grows in no other country besides Arabia, but it is not found in every part of it. About in the centre, upon a high mountain, he continues, is the country of the Atramites, a province of the Sabeans, from which the olibanum region is distant about eight days' journey. It is called Saba and is everywhere rendered inaccessible by mountains, a narrow defile, through which the export is carried on, leading into an adjoining province inhabited by the Mineans. In Saba itself were not more than 300 families, called the saints, who claimed the cultivation of olibanum as a right of heritage. When making the incisions in the trees, and while gathering the olibanum, the men were prohibited from having intercourse with women and from attending funerals. Notwithstanding the fact that the Romans carried on war in Arabia, none of them had ever seen an olibanum tree. When there was less chance of selling the olibanum, it was gathered but once in the year, but since the increase in the demand, it was gathered twice, first in the fall and again in the spring, the incisions in the trees having been made during the winter. The collected olibanum was brought upon camels to Sabota, where one gate was open for its reception; to turn from the road was prohibited under penalty of death. The priests took one-tenth by measure for the god Sabin, sales not being allowed until their claim was satisfied. The olibanum could be exported only through the territory of the Gebanites, whose King also levied tribute.
In ancient times olibanum was, without doubt, the most important perfume-substance. It was introduced into commerce by the Phoenicians, and, like many other substances, it received from them its name, which was adopted by other nations. Thus, the Hebrews called the tree lebonah, the Arabs, lubah, while the Greeks named it, λιβανός and the resin derived from it, the celebrated frankincense of the ancients, λιβανωτόςτς, Latin, olibanum. Regarding the mode of gaining the olibanum, some curious ideas prevailed in ancient times. Thus, Herodotus writes: "Arabia is the only country in which olibanum grows, as well as myrrh, cassia, cinnamon and lederum. With the exception of myrrh, the Arabs encounter many difficulties in procuring these products. Olibanum they obtain by burning styrax, for every olibanum tree is guarded by a number of small-sized winged serpents of a variegated appearance, which can be driven away by nothing but styrax vapors." According to Pliny, who gives a very full account of olibanum, Arabia felix received its by-name from the abundance of olibanum and myrrh found there. He states that olibanum grows in no other country besides Arabia, but it is not found in every part of it. About in the centre, upon a high mountain, he continues, is the country of the Atramites, a province of the Sabeans, from which the olibanum region is distant about eight days' journey. It is called Saba and is everywhere rendered inaccessible by mountains, a narrow defile, through which the export is carried on, leading into an adjoining province inhabited by the Mineans. In Saba itself were not more than 300 families, called the saints, who claimed the cultivation of olibanum as a right of heritage. When making the incisions in the trees, and while gathering the olibanum, the men were prohibited from having intercourse with women and from attending funerals. Notwithstanding the fact that the Romans carried on war in Arabia, none of them had ever seen an olibanum tree. When there was less chance of selling the olibanum, it was gathered but once in the year, but since the increase in the demand, it was gathered twice, first in the fall and again in the spring, the incisions in the trees having been made during the winter. The collected olibanum was brought upon camels to Sabota, where one gate was open for its reception; to turn from the road was prohibited under penalty of death. The priests took one-tenth by measure for the god Sabin, sales not being allowed until their claim was satisfied. The olibanum could be exported only through the territory of the Gebanites, whose King also levied tribute.
By the addition of fragrant substances to the oil, the sweet-scented ointment, myron, originated. While the anointing with simple oil evidently served as a hygienic measure after the bath, and especially for men in the gymnasium, and before a combat, with the Greeks, ointments were an article of luxury. In Socrates' time the use of sweet-scented ointments had reached such an extent, that Xenophon caused him to speak against it, but, as is the case with all such lectures against fashion, without the slightest success. In Athens the luxury was carried so far that the bacchanalians anointed each part of their body with a special ointment. The oil extracted from the palm was thought best adapted to the cheeks and the breasts; the arms were refreshed with balsam-mint; sweet marjoram supplied an oil for the hair and eyebrows; and wild thyme for the knee and neck. Although to us it would be repugnant to have the entire body anointed, in Athens it was considered beautiful to be glossy with ointments. It is said of Demetrius Phalereus, that in order to appear more captivating, he dyed his hair yellow, and anointed the face and the rest of his body.
From the Asiatics and Greeks the Romans also learned the use of ointments. Pliny cannot say at what time they were introduced in Rome, but states that after the conquest of Asia and the defeat of the King, Antiochus, in the year 565, after the building of Rome, the censors issued an edict prohibiting the sale of foreign ointments. However, this edict was of no use, and the practice spread more and more, Pliny speaking very bitterly about it. Regarding this extravagance in ointments, Plutarch says: "Frankincense, cinnamon, spikenard, and Arabian calamus are mixed together with the most careful art and sold for large sums. It is an effeminate pleasure and has spoiled not only the women but also the men, who will not sleep even with their own wives if they do not smell of ointments and powders." Plutarch further mentions an incident which must have created a sensation even in luxurious Rome, as otherwise it would scarcely have been chronicled for the benefit of posterity. Nero one day anointed himself with costly ointments and scattered some of them over Otho. The next day Otho gave Nero a banquet, and laid in all directions gold and silver tubes, which poured forth expensive ointments like water, thoroughly saturating the guests.
Directions for preparing ointments are contained in Theophrastus's work "On Perfumes," in Dioscorides's "Medica materia," and Pliny's "Historia naturalis." Dioscorides's receipts are the fullest. According to Pliny, a distinction was made between the juice and the body, the latter consisting of the fat oils and the former of the sweet-scented substances. In preparing the ointments, the oil together with the perfuming substances were heated in the water-bath. For instance, rose ointment was, according to Dioscorides, prepared by mixing 5½ lbs. of bruised Andropogon Schœnanthus with a little water, then adding 20½ lbs. of oil and heating. After heating the oil was filtered off, and the petals of one thousand roses were thrown into the oil, the hands with which the rose leaves were pressed into the oil being previously coated with honey. When the whole had stood for one night, the oil was strained off and when all impurities had settled, it was brought into another vessel and fresh rose leaves introduced, the operation being several times repeated. However, according to the opinion of the ancient ointment makers, no more odor was absorbed by the oil after the seventh introduction of rose leaves. To fix the odor, resins or gums were added to the ointments.
The consumption of perfume-substances by the ancient Romans must have been enormous. The trade of the ointment makers (ungentarii) was so extensive that the large street Seplasia in old Capua was entirely taken up by it, and the business must have paid well since the prices realized were very high. However, in ancient times the business cannot have been very agreeable, at least not in Greece, as shown by a passage in Plutarch's Life of Pericles: "We take pleasure in ointments and purple, but consider the dyers and ointment makers bondsmen and mechanics."
Depilatories were also known to the Romans, the agents employed being called psilothrum and dropax. They were of vegetable origin, but it is not exactly known from which plants they were derived.
We know but little regarding the use of perfumeries and cosmetics in the Middle Ages. In the wars during the migrations of the nations, but little thought was very likely given to them, but as soon as the nations became again settled and made sufficient progress in culture, the taste for perfumes and other pleasures of life no doubt returned. Our knowledge in this respect is limited to what is contained in the works of physicians of the first centuries. Later on we find receipts for cosmetics in the writings of Arabian physicians, such as Rhazes (end of the 9th to the commencement of the 10th century), Avicenna (end of the 10th to the commencement of the 11th century), and Mesuë (11th century). To the 11th century also belong the works of the celebrated Trotula, "De mulierum passionibus," "Practica Trotulae mulieris Salernitanae de curis mulierum," and "Trotula in utilitatem mulierum," all of which contain receipts for cosmetics. In the 14th century the most celebrated surgeon of the Middle Ages, Guy de Chanlios, did not consider it beneath his dignity to devote a section of his "Grande Chirurgie" to cosmetics. However, it was only in the 16th century that perfumes and cosmetics came again into prominent notice in Italy, which at that time was the country of luxury and art. Giovanni Marinello,[2] a physician, in 1562 wrote a work on "Cosmetics for Ladies," which he dedicated to the ladies Victoria and Isabella Palavicini. In the preface the author expresses the opinion that it is only right and pleasing to God to place the gifts bestowed by him in a proper light and to heighten them. He then proceeds to give perfumes for various purposes, aromatic baths to keep the skin young and fresh, means for increasing the stoutness of the entire body and of separate limbs, and others for reducing them. He further recommends certain remedies for making large eyes small, and small ones large. The chapter on the hair is very fully treated. To prevent the hair from coming out, rubbing with oil, and then washing with sorrel and myrobalan is recommended. For promoting the growth of the hair, the use of dried frogs, lizards, etc., rubbed to a powder, is prescribed. Means for making the hair long and soft and curly are also given, and others recommended for eyebrows and eyelashes. As depilatories lime and orpiment are prescribed. Paints are also classed among general cosmetics. Their use became at this time more and more fashionable, and not only the face, but also the breast and neck were painted.
Catherine of Medici and Margaret of Valois introduced these arts of the toilet into France. That country soon became the leader in this respect, and for many years the greatest luxury in perfumes and cosmetics prevailed there. The golden age for these articles lasted from the commencement of the seventeenth to the middle of the eighteenth century, during which time the mouche or beauty patch also flourished. "There were at that time hundreds of pastes, essences, cosmetics, a white balsam, a water to make the face red, another to make a coarse complexion delicate, one to preserve the fine complexion of lean persons and again one to make the face like that of a twenty-year old girl, an Eau pour nourir et laver les teints corrodés and Eau de chair admirable pour teints jaunes et bilieux, etc. Then there were Mouchoirs de Venus, further bands impregnated with wax to cleanse and smooth the forehead; gold leaf was even heated in a lemon over a fire in order to obtain a means which should impart to the face a supernatural brightness. For the hair, teeth and nails there were innumerable receipts, ointments, etc. However, of special importance were the paints, chemical white, blue for the veins, but, chief of all, the red or rouge, mineral, vegetable, or cochineal. The application of rouge was at that time no small affair, it was not only to be rouged, but the rouge had also to express something—Le grand point est d'avoir un rouge qui dise quelque chose. The rouge had to characterize its wearer; a lady of rank did not wear the rouge like a lady of the court, and the rouge of the wife of the bourgeois was not like either of them nor like that of the courtesan. At court a more intense rouge was worn, the intensity of which was still increased on the day of presentation, it being then Rouge d'Espagne and Rouge de Portugal en tasse. It may seem incredible, but for eight days a violet paint was used and then for a change Rouge de Serkis. Ladies, when retiring for the night applied a light rouge (un demi rouge), and even small girls wore rouge, such being the decree of fashion. The ladies dyed their eyebrows and eyelashes, and powdered their hair, both natural and false, for, about 1750, they commenced wearing wigs and chignons. Powdering was done partially for the purpose of dying the hair after dressing, and partially for decoration; white, gray, red and fiery red powders were in vogue."
Catherine of Medici and Margaret of Valois introduced these arts of the toilet into France. That country soon became the leader in this respect, and for many years the greatest luxury in perfumes and cosmetics prevailed there. The golden age for these articles lasted from the commencement of the seventeenth to the middle of the eighteenth century, during which time the mouche or beauty patch also flourished. "There were at that time hundreds of pastes, essences, cosmetics, a white balsam, a water to make the face red, another to make a coarse complexion delicate, one to preserve the fine complexion of lean persons and again one to make the face like that of a twenty-year old girl, an Eau pour nourir et laver les teints corrodés and Eau de chair admirable pour teints jaunes et bilieux, etc. Then there were Mouchoirs de Venus, further bands impregnated with wax to cleanse and smooth the forehead; gold leaf was even heated in a lemon over a fire in order to obtain a means which should impart to the face a supernatural brightness. For the hair, teeth and nails there were innumerable receipts, ointments, etc. However, of special importance were the paints, chemical white, blue for the veins, but, chief of all, the red or rouge, mineral, vegetable, or cochineal. The application of rouge was at that time no small affair, it was not only to be rouged, but the rouge had also to express something—Le grand point est d'avoir un rouge qui dise quelque chose. The rouge had to characterize its wearer; a lady of rank did not wear the rouge like a lady of the court, and the rouge of the wife of the bourgeois was not like either of them nor like that of the courtesan. At court a more intense rouge was worn, the intensity of which was still increased on the day of presentation, it being then Rouge d'Espagne and Rouge de Portugal en tasse. It may seem incredible, but for eight days a violet paint was used and then for a change Rouge de Serkis. Ladies, when retiring for the night applied a light rouge (un demi rouge), and even small girls wore rouge, such being the decree of fashion. The ladies dyed their eyebrows and eyelashes, and powdered their hair, both natural and false, for, about 1750, they commenced wearing wigs and chignons. Powdering was done partially for the purpose of dying the hair after dressing, and partially for decoration; white, gray, red and fiery red powders were in vogue."
Philip Augustus, in 1190, granted a charter to the French perfumers, who had formed a guild. This charter was, in 1357, confirmed by John, and in 1582 by Henry III., and remained in force until 1636. The importance of the craft in France is shown by the fact that under Colbert the perfumers or "parfumeurs-gantiers," as they were called, were granted patents which were registered in Parliament. In the seventeenth century Montpellier was the chief seat of the French perfumery industry; to-day it is Paris, and over fifty millions of francs' worth of perfumery are annually sold there. The parfumeurs-gantiers had the privilege of selling gloves of all possible kinds of material, as well as the leather required for them; they had the further privilege of perfuming gloves and selling all kinds of perfumes. Perfumed leather for gloves, purses, etc., was at that time imported from Spain. This leather was very expensive and fashionable, but on account of its penetrating odor its use for gloves was finally abandoned.
From the strength of the odor of a plant no conclusion can be drawn as to the quantity of volatile oil present. If this were the case, the hyacinth, for instance, would contain more oil than the coniferae, whilst in fact it contains so little that it can be separated only with the greatest difficulty. The odor does not depend on the quantity, but on the quality of the oil; a plant may diffuse but little odor and still contain much volatile oil. Of the various families of plants, the labiatae, umbelliferae, and coniferae are richest in volatile oils.
In every climate plants diffuse odor, those growing in tropical latitudes being more prolific in this respect than the plants of colder regions, which, however, yield the most delicate perfume. Although the East Indies, Ceylon, Peru, and Mexico afford some of the choicest perfumes, Central Europe is the actual flower garden of the perfumer, Grasse, Cannes, and Nice being the principal places for the production of perfume-materials. Thanks to the geographical position of these places, the cultivator, within a comparatively narrow space, has at his disposal various climates suitable for the most perfect development of the plants. The Acacia Farnesiana grows on the seashore, without having to fear frost, which in one night might destroy the entire crop, while at the foot of the Alps, on Mount Esteral, the violet diffuses a much sweeter odor than in the hotter regions, where the olive and the tuberose reach perfect bloom. England asserts its superiority in oils of lavender and peppermint. The volatile oils obtained from plants cultivated in Mitcham and Hitchin command a considerably higher price than those from other localities, this preference being justified only by the delicacy of their perfume. Cannes is best suited for roses, acacias, jasmine, and neroli, while in Nimes, thyme, rosemary, and lavender are chiefly cultivated. Nice is celebrated for its violets, while Sicily furnishes the lemon and orange, and Italy the iris and bergamotte.
Volatile Oils.—The volatile oils are either fluid (actual volatile oils) or solid (varieties of camphor) or solutions of solid combinations in fluid. The latter, on exposure to low temperatures, separate into two portions, one solid, called stearoptene, and the other liquid, called elæoptene. The boiling point of the volatile oils is considerably higher than that of water, but when heated with water they pass over with the vapors. Upon paper, fluid volatile oils produce grease spots, which differ, however, from those caused by fat oils in that they gradually disappear at an ordinary temperature, and rapidly by gentle heating. Most volatile oils are insoluble, or only with difficulty and sparingly soluble, in water, but they impart to the latter their odor and taste. They are readily soluble in alcohol, ether, chloroform, bisulphide of carbon and petroleum-ether, and miscible in every proportion with fats and fat oils. By their solubility in alcohol they differ from most fat oils. When freshly prepared many volatile oils are colorless, but soon turn yellow; some, however, show a distinct color even when fresh. They ignite with greater ease than fat oils and burn with a fierce smoky flame depositing a large amount of carbon. They exhibit a great tendency to absorb oxygen from the air and to gum, the influence of light promoting the process. In specific gravity they range from about 0.75 to 1.17, most of them being specifically lighter than water. Most bodies, under otherwise equal conditions, show always exactly the same specific gravity, the variations being so slight that they may be justly ascribed to errors of observation. However, one and the same volatile oil frequently shows such variations in specific gravity, that we are forced to ascribe this phenomenon to alterations in the constitution of the oil itself. For the exact determination of the specific gravity of a volatile oil, it should, therefore, be subjected to examination immediately after its preparation from the plant or vegetable substance, which should be as fresh as possible. The influence of light upon volatile oils is best shown by the following interesting experiment: If certain volatile oils are distilled in a vacuum or over burnt lime in a current of carbonic acid, it is no longer possible to distinguish, for instance, oil of lemon from oil of turpentine; however, by again exposing the oils to the air, they reacquire their characteristic odor.
On account of the facility with which most of the volatile oils absorb oxygen, oils originally free from oxygen are frequently a mixture of hydrocarbons and combinations containing oxygen. The volatile oils varying so much in their physical as well as their chemical properties, a suitable classification of them has thus far been unsuccessful.
All the terpenes occurring in the various oils are combinations having the formula C10H16, or polymeric with it, C15H24, C20H32, etc. These terpenes exhibiting certain deviations in regard to their properties, odor, specific gravity, and boiling points, nearly as many terpenes as there are volatile oils have been distinguished. It is, however, very likely that these deviations may be traced back to fortuitous circumstances, for example, to the admixture of foreign substances occurring together with the terpenes, and that, by a more accurate examination, the number of terpenes entitled to be considered pure chemical combinations will be considerably reduced. By Wallach's labors, the identity of several terpenes formerly considered distinct, has already been established, whilst many others have been found to possess properties in common.
By expression a turbid milky fluid is obtained, which consists of the volatile oil and aqueous substances. The latter are a solution of various extractive substances and salts in water. This fluid, as it runs from the press, is received in tall, narrow, glass vessels and brought into a cool place for clarification. This frequently requires several days, three distinct layers being generally distinguished. On the bottom is a mucous layer consisting of cell substances carried along by the liquid bodies. Over this is a clear fluid consisting of a solution of extractive substances, vegetable albumen, and salts, and upon this floats the volatile oil, being specifically the lightest body, which, by its greater refractive power, can be clearly distinguished from the aqueous fluid.
There are two methods of obtaining the oil entirely clear, viz., filtration and distillation. Filtration is the cheaper process, but requires special precautions to exclude the air as much as possible to prevent the oil from undergoing injurious changes. By arranging the filtering apparatus so that the oil always comes in contact with only the same quantity of air, the injurious action of the oxygen is reduced to a minimum. It is self-evident that the apparatus should not be placed in the sun, but in a semi-dark, cool place.
A filter of simple construction, and performing excellent service, is shown in Fig. 1. It consists of a large glass bottle, F, hermetically closed by a doubly-perforated cork. The neck of the glass funnel T, the upper rim of which is ground smooth, is placed in one of the holes, and a glass tube, r, bent at a right angle, is fitted into the second hole. A thick wooden lid, with a rubber ring on the lower side, is placed upon the funnel, thus closing it air-tight. In the centre of the lid is fitted a glass tube, r´, also bent at a right angle, which is connected with the tube r, by a rubber hose, k. After the funnel has been provided with filtering paper and the oil to be filtered, the lid is placed upon it, and must not be removed, except for the purpose of pouring more oil into the funnel. The air in the bottle F is displaced by the oil dropping into it, and escapes through r, k and r´ into the funnel, and thus only the air in the bottle and funnel can act upon the oil.
Distillation.—There are at present two methods in use. The one is founded upon the direct action of the heat, the other upon the use of steam. The first was formerly in general practice, and is still largely employed in France and England, and to a limited extent in this country. It is, however, very deficient in many respects. As the stills must necessarily be of small capacity, only small quantities can be distilled at one time, and the oils very rarely possess the peculiar odor due to them, and sometimes the odor is even altered. In mixing too little water with the materials to be extracted, there is danger that empyreumatic oils will be formed; a large quantity of water, on the other hand, is of disadvantage, in so far as in case the perfume-materials contain little oil, only a perfumed water, but no oil, will be obtained. In order to avoid these inconveniences, or, at least, to do away with some of them, another plan was devised. The materials to be distilled were spread upon sieves, which were suspended in the upper part of a still, so that they might be penetrated from below. It is true no scorching is possible in this case, as was in the other process when the heating was continued after all the water had evaporated, and the oil retains its proper color, but by this method only small quantities can be extracted at a time. The still generally used for distillation with direct heat resembles so much an ordinary whiskey still as to need no further description here.
For the accurate determination of the percentage of volatile oil a vegetable substance will yield, or to obtain the oil from very costly raw materials, the small glass apparatus, Fig. 2, is used. The flask A, with a capacity of up to 5 or 6 quarts, serves for a still. In the tube t, shaped like the neck of a bottle, is inserted by means of a cork, a funnel tube, l, reaching to the bottom of the flask. The neck of the flask passes into the cooling pipe, which lies in a so-called Liebig cooler. This consists of a wide-glass tube, C, into the lower end of which, at h, flows cold water from the reservoir D, displacing the heated water at g. The lower end of the cooling pipe is connected with the neck-shaped tube v, under which stands the vessel for the reception of the distillate. To prevent the cracking of the flask, which might readily happen with the use of direct heat, it is placed in a vessel filled with sand or water.
An improved apparatus for distilling dry substances by steam has been patented in Germany by Messrs. Schimmel & Co., of Leipzic. The tall conical column at the left (Fig. 6) is the still. About eight inches from the bottom is a perforated diaphragm or false bottom, upon which the material to be distilled is placed by introducing it through the still-head. A perforated coil below the diaphragm projects steam upwards through the mass, which is occasionally agitated from without by means of a horizontal stirring apparatus indicated by the two crosses. Any condensed water which may run back is converted into steam by the heating coil at the bottom. Meanwhile, the mass itself is heated by a long coil lining the body of the still and carrying steam at a high pressure. Whatever of volatile oil is carried forward by the steam passes through the still-head into the cooler on the right, where both oil and steam are condensed, and from where they flow through a small funnel tube into three successive receivers, which are arranged like Florentine flasks, and which retain the volatile oil that has separated. From the last receiver the water, which is still impregnated with oil, enters another reservoir, shown in the illustration only by dots, and from there it flows into a small globular still situated underneath; in which, by means of steam, nearly all the oil still retained is again volatilized with the steam of the water and both again conducted to the cooler.
Separation of the oil and water.—As previously mentioned the specific gravity of most volatile oils is less than that of water. This behavior is utilized for the separation of the oil and water, by means of a so-called Florentine flask (Fig. 7). It consists of a glass flask provided near the bottom with a pipe, a, rising vertically to near the neck c of the flask where it is bent downwards as shown in the illustration. The mixed liquid of water and oil drips from the cooling pipe into the flask, and the water w, being specifically heavier, separates from the oil floating on the top, and gradually ascends in the pipe a, finally flowing over at d. Oils specifically heavier than water are caught in receivers provided with a discharge-pipe near the mouth of the flask as shown in Fig. 8.
Most volatile oils are obtained by distillation, but this method is not practicable for separating the odoriferous principle of many of the most sweet-scented and delicate flowers, partially because the flowers contain too little oil, and partially because the oil would lose in quality if obtained by distillation.
Extraction.—For obtaining the volatile oils by extraction various solvents such as ether, bisulphide of carbon, etc., may be employed. Carefully rectified petroleum-ether is very suitable for the purpose. It completely evaporates at about 122° F., and when sufficiently purified does not possess a disagreeable odor. The process of extraction is briefly as follows: The material to be extracted is treated in a digester with petroleum-ether or one of the above-named solvents. The solution is then drawn off and the solvent evaporated in a still. The recondensed solvent flows immediately back into the digester and further extracts the material contained therein. The operation is repeated until nothing soluble remains. In practice some difficulties are, however, connected with this process since, besides the volatile oils, resins, and coloring and extractive substances are dissolved, which have to be removed, as well as the last traces of the solvent, as otherwise the oil would acquire a foreign odor. Further the solvents mentioned are very volatile and inflammable, requiring the greatest precautions as regards fire. For these reasons the extraction process is not suitable for many purposes, and though at first great hopes were entertained in regard to it, its use is limited to substances with a large content of volatile oil.
On account of the great volatility of bisulphide of carbon, considerable loss would, however, be incurred by the above-mentioned admission of air. To avoid this, the reservoir serving for the reception of the condensed bisulphide of carbon and aqueous vapor is closed, and connected by a pipe with a long, narrow, horizontal cylinder half filled with oil, and provided with a fan-shaft. The vapors of bisulphide of carbon entering the cylinder from the reservoir are absorbed, together with the air by the oil, the surface of which is constantly agitated by the fan-shaft, while the air, rendered entirely inodorous, passes out at the other end. The bisulphide of carbon is finally separated from the oil by distillation and again used.
The saturated oil solution is subjected to distillation, which is readily effected in Heyl's apparatus, Fig. 15. The lower part of the still A of boiler plate is surrounded by the steam-jacket B, into which steam is admitted through C and the condensed water discharged through D. The concentrated oil solution runs from a reservoir, standing at a higher level through the pipe E into the still, the admission of a sufficient quantity being indicated by the gauge F. The bisulphide of carbon brought to the boiling point (114° F.) by the steam introduced into the jacket, vaporizes quickly; the vaporization being still more accelerated by revolving the stirrer H, by means of the crank G. The vapors of bisulphide of carbon escape through four openings in the upper part of the still, into a capacious worm, the lower part of which enters, under water, a reservoir.
Maceration or infusion.—This process is employed for flowers with an inconsiderable content of volatile oil or whose odoriferous substance would suffer decomposition or alteration by distillation. The process is founded on the affinity of odoriferous substances for fatty bodies which, when impregnated with them, are called pomades. These are afterwards made to yield the aroma to strong alcohol, so that finally there is obtained a solution of the volatile oil in alcohol from which the pure oil is obtained by distilling off the alcohol. The fat used, olive oil, lard, etc., should be entirely neutral, i. e., free from every trace of acid. The fats are purified by treating them several times in the heat with weak soda-lye and then washing carefully with water until the last traces of the lye are removed, and the fat shows no alkaline or acid reaction.
Experience, however, has shown that volatile oils prepared by this process possess a finer odor the shorter the time the flowers remain in contact with the fat. Piver has devised an apparatus which reduces the time of maceration to the shortest period possible. The kettle to the left, Fig. 16, supplies the fat heated to the proper temperature, which circulates slowly through the macerating tank, in which a constant temperature of 149° F. is maintained by means of a steam pipe. The macerating tank is divided into compartments, in which baskets containing the vegetable substance to be extracted are suspended. The basket on the left contains the substance which has passed through all the compartments; it is from time to time removed, filled with fresh substance, and then attached to the right, the other baskets being moved to the next compartment to the left. In this way the fresh substance has to traverse each compartment from right to left, while the fat flows slowly from left to right, and saturated with the perfume of the substance collects in the tank on the extreme right.
The process of absorption, or "enfleurage," as it is called by the French, is chiefly made use of for procuring the odoriferous principle of very delicate flowers, the delicious odor of which would be greatly modified, if not entirely spoiled, by the application of heat. The older apparatus employed for the purpose consists of a number of shallow wooden frames of about 15×18 inches, inclosing at half their depth a sheet of glass. The edges of the frame rise about an inch above each surface of the glass, and, being flat, the frames stand securely upon one another, forming often considerable stacks. These frames are called "chassis," those just described being termed "chassis aux vitres," or "chassis aux pomades," to distinguish them from a different form, which is used where oil has to be submitted to the process of absorption. The process in the case of pomade is as follows: Each sheet of glass is uniformly coated with a thin layer of purified grease, care being taken that the grease does not come in contact with the woodwork of the frames. The flowers are then thinly sprinkled, or rather laid, one by one, upon the surface of the fat, where they are allowed to remain one or two days, when they are removed and replaced by fresh ones. The operation is thus continued for twenty-five or thirty days, until the fat is saturated with aroma. The frames charged with fat and flowers are stacked one upon the other, forming, in fact, a number of little rectangular chambers.
Odor and taste are so characteristic for every volatile oil as to suffice in most cases. For testing as to odor, bring a drop of the oil to be examined upon the dry palm of one hand and for some time rub with the other, whereby the odor is more perceptibly brought out. To determine the taste, vigorously shake one drop of the oil with 15 to 20 grammes of distilled water and then test with the tongue.
The presence of castor oil can be accurately determined by bringing the residue from the watch-crystal into a test-tube by means of a glass-rod, and compounding it with a few drops of nitric acid. A strong development of gas takes place, after the cessation of which, solution of carbonate of soda is added as long as there is any sign of effervescence. If the added oil was castor oil, the contents of the test-tube will show a peculiar odor due to œnanthylic acid formed by the action of nitric acid upon castor oil.
Detection of alcohol or spirit of wine.—Independent of the alcohol added to assist the preservation of some oils, adulteration with alcohol frequently occurs, especially in expensive oils. With a content of not more than 3 per cent. of alcohol, it suffices to allow one to two drops of the suspected oil to fall into water. In the presence of alcohol, the drop becomes either immediately surrounded with a milky zone, or it becomes turbid or whitish after being for some time in contact with the water. Dragendorff's test is based upon the fact that oils, which are hydrocarbons, suffer no change by the addition of sodium (ten drops of oil and a small chip of sodium), while oils containing hydrocarbons and oxygenated oils cause with sodium a slight evolution of hydrogen gas, and suffer but a slight change during the first five to ten minutes of the reaction. If, however, the oil is adulterated with alcohol, not only a violent evolution of hydrogen gas takes place, but the oil in a short time becomes brown or dark brown, thickly fluid or rigid.
The above-mentioned oils may, however, be rendered fit for the tannin test by mixing them with double their volume of benzine or petroleum-ether, and allowing the mixture to stand for two or three days. If, however, the oils contain much alcohol, the tannin is dissolved. The use of powdered tannin is not advisable, because it generally deposits in a thin layer on the bottom, and its alteration is not so perceptible. If, for practical reasons, a content of 0.5 per cent. anhydrous alcohol might be accepted as permissible in a volatile oil, the tannin test would have to be so modified as to mix 10 drops of the oil with a piece of tannin the size of two peas, and allow the whole to stand for one hour. In this time the above-mentioned content of alcohol would yield no result.
Detection of benzine.—An adulteration with benzine can be readily detected only in oils specifically heavier than water. The separation of benzine is effected by distillation from a small glass flask in the water bath. The distillate together with an equal volume of nitric acid of 1.5 specific gravity is gently heated in a test-tube. A too vigorous reaction is modified by cooling in cold water, and a too sluggish action quickened by gentle heating (dipping in warm water). If the mixture has a yellow color, dilute it with water, shake with ether, mix the decanted ethereal solution with alcohol and hydrochloric acid, add some zinc and place the whole in a lukewarm place to convert the nitrobenzol formed into aniline. After evolution of hydrogen is done, neutralize with potash lye, shake, take off the layer of ether, let the latter evaporate and add to the residue a few drops of calcium chloride solution. If benzine is present, a blue-violet color reaction takes place.
Adulterations with alcohol, chloroform, and benzine are quantitatively determined by bringing a weighed quantity of the oil into a glass flask so that it occupies about four-fifths of the volume of the flask. Place upon the flask a cork through which has been passed a glass-tube bent at a right angle and provided with a cylindrical glass vessel serving as a receiver and heating in the water bath. If the distance from the level of the oil to the angle of the glass tube in which it inclines downwards, amounts, for instance, to 4.72 inches, and the neck of the flask up to its angle is 2.75 inches high outside of the direct effect of the heat of the water bath, only the above-mentioned adulterants distill over, while the vapor of the volatile oil condenses at a height of 2.75 inches and flows back into the flask. The distillate is weighed and examined as to its derivation. First add one cubic centimeter of it to two or three cubic centimeters of potassium acetate solution of specific gravity 1.197 and shake moderately. If a clear mixture results, alcohol alone is present. If, however, the mixture is not clear, and the distilled fluid sinks down and collects on the bottom of the test-tube, chloroform is very likely present, and if it remains floating upon the acetate solution, benzine. Next bring two to three centimeters of the distillate into a test-tube and add a piece of sodium metal, the size of a pea. If violent foaming, i. e., an evolution of gas, takes place, alcohol is certainly present, and possibly also chloroform and benzine towards which sodium is indifferent. However, in the presence of benzine, the sodium solution would be colorless, and in the presence of chloroform, yellowish and turbid. In case the sodium produces no reaction and alcohol is, therefore, not present, add an equal volume (two to three cubic centimeters) of anhydrous alcohol, and after moderately shaking allow the solution of the sodium and the evolution of gas to proceed, whereby benzine produces a nearly colorless, turbid fluid, and chloroform a yellowish, milky one. Now dilute the fluid with an equal or double volume of water, shake and allow the mixture to stand quietly. In the presence of benzine a colorless, turbid layer collects on the bottom of the fluid, while that collecting in the presence of chloroform is yellowish. In the latter case, i. e., in the presence of chloroform, the aqueous filtrate yields with lead acetate solution a white precipitate (lead chloride and lead hydroxide). The adulterant having thus been recognized, further particulars are learned from the specific gravity of the oil as well as of the distillate.
In place of iodine, Rudolph Eck recommends a very dilute alcoholic iodine solution, which is not discolored by oils of turpentine, while other oils discolor it. Dissolve a drop of the oil to be examined in 3 cubic centimeters of 90 to 100 per cent. alcohol, and add a drop of the iodine solution. The latter is not discolored in the presence of an oil of turpentine. There are also, however, several volatile oils, which do not discolor the iodine solution. Mierzinski mentions the following: All cold-expressed oils from the Aurantiaceæ, further oils of coriander, caraway, galanga, rue, sassafras, rose, rosemary, anise-seed, fennel, calamus, neroli, angelica, wormwood. Hence, this reaction cannot be relied upon.
II. Hoppe's nitroprusside of copper test.—This test sometimes gives good results, but only with hydrocarbons absolutely free from oxygen and oxygenated oils. It is, therefore, not suitable for oils derived from the Aurantiaceæ. The process is as follows: Add to a small quantity of the oil to be examined in a perfectly dry test-tube, 2 to 5 milligrammes of pure nitroprusside of copper previously thoroughly dried and finely pulverized, shake vigorously and gradually heat to boiling. After boiling for a few seconds allow to cool. If the oil is free from oil of turpentine, or another oil containing no oxygen, the precipitate formed is brown, black, or gray, and according to the quantity of the reagent added and the original color of the oil, the supernatant oil will be differently colored and appear more or less dark. If, however, the oil is adulterated with oil of turpentine, the precipitate formed shows a handsome green or blue-green color, while the supernatant oil retains its original color or at the utmost acquires a very slightly darker one. The longer the oil is allowed to stand after settling, the more distinct and beautiful the color of the oil and of the precipitate appears. For the establishment and certain recognition of very small quantities of oil of turpentine in oxygenated oils, it is best to first add very little of the nitroprusside of copper to the oil to be tested, and a larger quantity only after being convinced either of the purity or adulteration of the oil. This is done to be able, on the one hand, better to judge the reaction, if the oil is pure, and, on the other, if it is adulterated, to establish such adulteration with certainty and to approximately estimate the quantity of oil of turpentine present. The less nitroprusside of copper is used, the better small quantities of oil of turpentine can be detected.
If these oxygenated oils are mixed with oils free from oxygen, for instance, oil of turpentine, they show exactly the same behavior as oils free from oxygen; the nitroprusside of copper is not decomposed and retains its gray-green color. If, for instance, oil of cloves is mixed with oil of turpentine, the red coloration by nitroprusside of copper does not appear.
IV. Hager's guaiacum reaction[3] serves for the detection of oil of turpentine in a volatile oil. By pouring upon as much guaiacum, freshly powdered, as will lie upon the point of a small knife, in a test-tube 1 cubic centimeter (25 drops) of spike oil, and heating nearly to boiling over a petroleum lamp, the oil after being removed from the flame and allowing the undissolved resin to settle, shows a yellow color. By now pouring upon an equal quantity of guaiacum in another test-tube 25 drops of spike oil and 5 drops of rectified oil of t from the flame shows a dark violet color. Various other oils behave in the same manner as spike oil, and hence a content of oil of turpentine can be readily detected in them. Other oils do not exhibit this behavior; but this can be remedied by adding, in testing for oil of turpentine, a few drops of an oil of the first class.
c. Oils with a content of oil of turpentine, which remain indifferent towards guaiacum.—To such oils, if to be tested for oil of turpentine, with the assistance of the guaiacum reaction, a few drops of an oil of the second class have to be added.
Barenthin has in this manner determined the iodine number of several volatile oils; other experimenters, however, for instance, Kremel and Davies,[4] have found different numbers for the same oils, so that this method requires further thorough examination before it can be classed as available.
The execution of the method is as follows: Dissolve 1 gramme of the oil to be examined in 2 to 3 cubic centimeters of 90 per cent. alcohol freed from acid, compound the solution with a few drops of phenol-phthalein solution, and titrate the free acid with ½ normal alcoholic potash lye. The milligrammes of caustic potash used are designated the "acid number." After having thus determined the content of acid, add to the same solution 10 cubic centimeters of the same potash lye, heat for ¼ hour upon the water bath, and then titrate back the excess of potash lye with ½ normal hydrochloric acid. In this manner the "saponification number" is obtained. (In some cases when the final reaction is not plainly perceptible, it is advisable to correspondingly dilute with water after heating the alcoholic fluid.) The saponification number, less the acid number, gives the "ether or ester number."
VII. F. R. Williams has recently endeavored to utilize for testing volatile oils Maumené's test, which is based upon the increase in temperature produced in oils by concentrated sulphuric acid, and which gives valuable points for the examination of some fat oils. Of course, the large quantities of oil otherwise prescribed cannot be used. While for the examination of fat oils 50 grammes of oil are mixed with 10 cubic centimeters of concentrated sulphuric acid in a beaker glass wrapped around with cotton, Williams could use only six cubic centimeters of volatile oil. They were brought into a very small beaker glass enveloped in cotton. After reading off the temperature, twelve cubic centimeters of concentrated sulphuric acid were added and the whole stirred with the thermometer until the temperature no longer rose. Numbers were in this manner obtained which might in some cases, for instance, cassia oil, furnish guiding points for judging the purity of the oil.
By withdrawing the chlorine and one atom hydrogen from the benzyl chloride and introducing for it one atom oxygen, the benzyl chloride is converted into benzaldehyde. This conversion is readily effected by continuously boiling, best with the introduction of carbonic acid, 1 part of benzyl chloride with 1½ parts of lead nitrate and 10 parts of water, and finally distilling the benzaldehyde off by steam. The decomposition takes place according to the following equation:—
An adulteration with nitrobenzole and other volatile oils is recognized by mixing 2 drops of the oil with 100 drops of distilled water, and shaking vigorously. Pure oil must completely dissolve. However, the test yields accurate results only with the use of actually pure distilled water and by accurately observing the above-mentioned proportions. If to 5 cubic centimeters of 90 per cent. alcohol and an equal quantity of distilled water in a test-tube, 10 drops of the oil be added, and, after closing the tube with the finger, mixture be effected by gently turning the tube twice upside down, a clear solution will immediately result if the oil is pure. If, however, it contains nitrobenzole, even only 1 per cent., the latter separates, at first rendering the fluid turbid, but in the course of a minute, when gently agitated, it floats in the form of minute drops upon the fluid, while, when at rest, these drops collect to larger ones on the bottom of the test-tube. If the oil becomes only turbid, adulteration with other volatile oils is indicated. Another test, given by Wagner, is based upon the difference in the specific gravity of mixtures of oil of bitter almonds with oil of mirbane. The specific gravity of commercial oil of bitter almonds varies between 1.040 and 1.043 and that of oil of mirbane between 1.180 and 1.201.
Angelica oil is obtained by distillation with water from the root of Angelica Archangelica L., natural order Umbelliferae. The oil is lighter than water, possesses the spicy odor of the root and an aromatic pungent taste. It consists mostly of a terpene which turns the plane of polarization to the right, and boils at 320° F.
Freshly prepared anise-seed oil is colorless or straw-yellow, has the odor of anise and a sweetish taste, leaving a burning sensation upon the tongue. It is thinly fluid at 68° F., but commences to congeal at a somewhat lower temperature, and the sooner the more stearoptene it contains. Good oil should become solid at from 57.2° to 60.8° F. It has a specific gravity of 0.980 to 0.995 at 59° F. The specific gravity varies with the content of stearoptene; the greater the latter the higher the specific gravity. Good anise-seed oil contains 5 to 10 per cent. of terpene and 90 to 95 per cent. of a stearoptene, called anethol, C10H12O, on which the value of the oil depends. The anethol can be separated from the oil by cooling to 32° F., and forms colorless crystals. It has an agreeable odor and intensely sweet taste, is sparingly soluble in water, but readily in alcohol, ether, and other solvents of volatile oils. Good anethol has a specific gravity of 0.986, and melts at 69° to 70° F. By frequent contact with the air a small portion of the anethol is oxidized, very likely to anisaldehyde. By this process the specific gravity is raised and the melting point lowered.
Bayberry oil, or oil of bay leaves, is extracted by distillation from the leaves of Myrcia acris or the bayberry tree. Many varieties of the tree exist throughout the West Indies, which are scarcely to be distinguished botanically, but have quite a different odor from that of the genuine tree. Great care must, therefore, be taken in the collection of the leaves which are to be used, as the admixture of a small quantity of the other leaves may entirely spoil the product of distillation. Two oils are obtained, a light oil of specific gravity of 0.870 to 0.990, and a heavy oil with specific gravity 1.023 to 1.037. When first distilled the oil is colorless, but by exposure to the air quickly acquires a yellowish tint and, if the exposure be continued, becomes quite dark in color. The odor of the freshly-distilled oil is rank, but in the course of from three to six months it becomes mellow, and ripens into the agreeable fragrance so much liked in the best specimens of bay-rum. The oil is soluble in all proportions in 95 per cent. alcohol, also in ether and petroleum benzine. Its chief use is for the preparation of bay-rum.
Bergamot oil may be tested as to its purity by mixing it with alcohol. It becomes pale gray-yellow, forms a sediment which adheres firmly to the vessel and, on shaking, floats about in the form of flakes. After two days the sediment is inconsiderable and difficult to divide into flakes in the clear yellow fluid by shaking. The oil is frequently adulterated with alcohol. To detect such adulteration, Righini recommends the following method: Mix 15 parts of the oil with a like quantity of pure olive oil or oil of sweet almonds. If alcohol is present, it immediately separates, like water, from the fat oil; if no separation takes place the oil is not adulterated with alcohol. The tannin test also gives reliable results. In storing oil of bergamot great care must be exercised to exclude air and light, as it is one of the most changeable oils and soon acquires an odor resembling that of turpentine.
It is claimed that an artificial cajeput oil is often prepared from camphor and rosemary oil, the green color being obtained by distillation with milfoil. The presence of camphor may be readily determined by thoroughly triturating a few drops of the oil with sugar and then dissolving in water, whereby the particles of camphor separate in the form of white flakes upon the surface.
The purity of caraway oil is recognized by its dissolving clear in equal parts of 90 per cent. alcohol. If such is not the case, the oil contains either an admixture of oil of turpentine or does not possess the full normal content of carvol. Pure caraway oil does not detonate with iodine, which is the case with oil containing oil of turpentine.
Cherry-laurel oil (oleum laurocerasi) is the volatile oil, which contains prussic acid, obtained from the leaves of the cherry-laurel (Prunus laurocerasus, L.). Like bitter almonds, the leaves contain some amygdalin. Hence they are macerated with water and allowed to stand in a warm place for 24 hours. By subsequent distillation a volatile oil is obtained which closely resembles oil of bitter almonds, but differs in some respects. It is colorless or yellowish, rarely reddish, and of specific gravity 1.05 to 1.06. In its behavior towards air, solvents, and reagents, it does not essentially differ from oil of bitter almonds.[5]
Ceylon cinnamon oil (oleum cinnamoni ceylonici).—Formerly this oil was exclusively distilled from chips and waste of the genuine cinnamon bark of the Cinnamonum ceylonicum, Nees, and came into commerce from Ceylon. However, the fabrication of the oil from cinnamon waste or chips is now extensively carried on in Germany, and this oil, being prepared with the assistance of more perfect apparatus, has almost entirely supplanted that exported from Ceylon.
Cassia oil (oleum cassiæ).—In China and Cochin China this oil is obtained by distillation from the bark, unripe fruits, buds, and other waste of the Cinnamonum cassia or Cinnamonum aromaticum, Nees, a tree indigenous to those countries. It has a pale yellow color, which in time becomes brown. It is thickly-fluid, of specific gravity 1.05 to 1.07, and possesses a sweet taste with an acrid after-taste. Like cinnamon oil, it consists chiefly of cinnamaldehyde, but contains no eugenol, and hence can be readily distinguished from Ceylon oil by the above-mentioned reaction. One part of pure cassia oil dissolves in two parts of 80 per cent. alcohol.
Rectified citron oil is colorless, of an agreeable penetrating odor and acrid taste, and very sensitive to light and air. By exposure to light it turns yellow, and if air be admitted at the same time, it is first converted into a fluid which, on account of its content of ozone, possesses strong bleaching powers. The oil at the same time acquires a disagreeable odor, resembling that of oil of turpentine, and is finally converted into a resinous mass.
According to experiments by Schimmel & Co., pure citronella oil must give a clear solution, when 1 part of the oil is vigorously shaken with 10 parts of 80 per cent. alcohol. If, in executing the test, the kind of turbidity is observed, and whether the portion insoluble in alcohol separates, after standing, upon the surface or on the bottom of the fluid, and further, if the above-mentioned quantity of alcohol is not added at one time, but at first only 1 or 2 parts of it, a conclusion may be drawn as to the kind and quantity of the adulterant.
Cloves, oil of (oleum caryophylli), is obtained by distillation with steam, or by extraction from the cloves of commerce, which are the dried unexpanded flower buds of Caryophyllus aromaticus, L., or the clove tree. Oil of cloves, when fresh, is almost colorless, but on exposure to air acquires a brownish coloration and a thickly fluid consistency. It has the aromatic taste and odor of cloves, and a specific gravity of 1.300 to 1.065. It frequently shows a slightly acid reaction, boils at 482° F., and congeals at 4° F. It is readily soluble in alcohol, ether, and strong acetic acid. It consists of a terpene (C10H16) and eugenol (C10H12O2), the odor of the oil being due to the latter. The terpene has a specific gravity of 0.918, and in distilling passes over first (light oil of cloves). The eugenol, when fresh, is colorless, has the odor and taste of cloves, a specific gravity of 1.063 at 65° F., boils at 487.4° F., is insoluble in water and glycerin, but soluble in alcohol, ether and glacial acetic acid. Its alcoholic solution is colored magnificently blue by ferric chloride. If in an alkaline solution it is oxidized with potassium permanganate, vanillin being formed.
To test the value of oil of cloves, introduce, according to Stohman, into a graduated glass cylinder 10 volumes ether, 10 oil of cloves, and 30 of a 10-per cent. soda solution. After vigorous shaking, the eugenol dissolves; the increase in volume of the aqueous fluid is then proportional to the quantity of eugenol present. For more exact determinations, dissolve a weighed quantity of oil, repeatedly shake the aqueous fluid with ether to remove the terpene, then decompose the eugenol-sodium with dilute sulphuric acid, dissolve the separated eugenol in ether and weigh after evaporating the ethereal fluid. Good oil of cloves does not contain less than 80 per cent. of eugenol, and frequently 90 per cent. or more.
When not rectified, eucalyptus oil is mostly yellowish or bluish. In a rectified state it is colorless, clear, limpid, lighter than water, of a strong odor, and acrid taste. The oil from eucalyptus globulus has a specific gravity of 0.900 to 0.925, and dissolves in every proportion in 90 per cent. alcohol. It is optically inactive or turns the plane of polarization slightly to the right. On standing with sodium it acquires a yellowish coloration, and does not detonate with iodine. The oil from eucalyptus australe has a specific gravity of 0.86 to 0.87, and is but sparingly soluble in 90 per cent. alcohol, so that even a solution prepared in the proportion of 1:15 is turbid. It turns the plane of polarization strongly to the left; acquires, on standing with sodium, a red coloration, and detonates with iodine.
French and African geranium oils (oleum geranii) are obtained by distillation with water from the leaves of various species of pelargonium. Many different kinds of this oil are found in commerce. The finest and most expensive are the Spanish and French geranium oils, so-called rosé, which are distinguished by their fine odor, closely resembling that of rose oil. They are derived from Pelargonium radula, and are either yellowish, brownish, or pale green, the brownish oils being preferred. It congeals at 60.8° F. and turns the plane of polarization to the right. Another good geranium oil is the African, which is chiefly prepared in Algiers from Pelargonium roseum, Wildenow, and P. odoratissimum, Aiton. It closely resembles the French oil, but turns the plane of polarization to the left.
Jasmine oil or oil of jessamine, from the flowers of Jasminium officinale, L., and J. grandiflorum.—The oil is exclusively obtained by the absorption process, and is the most prized by the perfumer. It is, however, exceedingly rare on account of the enormous cost of its production. The extract of jasmine, the "essence de jasmine" of the French manufactories, is a solution of the oil, as obtained by extraction with lard or beef suet, in strong spirit of wine. The odor of jasmine oil is so peculiar that it is without comparison, and as such cannot be imitated.
Lemon oil (oleum limonis) is obtained by various processes from the rinds of lemons. The best and most delicately-scented oil is obtained by the so-called sponge process in use in Southern Italy and Sicily. The rinds are soaked from fifteen to twenty-five minutes in water, to which sometimes a little soda is added. They are taken up singly in the right hand and the outer surface of each is firmly pressed against a large and rather hard-grained sponge held in the left hand and secured by a strap. Two or three sharp turns of the wrist impart what may be called a screw-pressure to the rind, thus effectually fracturing the oil cells, the sponge absorbing the contents. The sponge is constantly held over an earthen jar and occasionally squeezed into it. The fluid in the jar quickly separates into three different products—the dregs or deposit of mucilaginous and cellular matter, some fruit juice, and the pure oil, which floats on the top. The latter, when bright and clear, is passed, by means of a small glass siphon, into the cans of thin copper, in which, after sealing, it is stored away for export.
Another method of expressing the oil is that of the écuelle à piquer, much used in the region about Nice. The oils obtained by this method, which are also of a very fine quality, are marked essence à l'écuelle or au zeste. The apparatus consists of a round shallow pan of copper or brass, having a receptacle for the oil at its lowest part and a lip on one side for pouring, and studded on its concavity by strong blunt spikes. The workman takes the fruit and rolls it gently but quickly around the inside of the écuelle; the spikes prick the oil sacs, whereupon the oil, running down the spikes and the concavity of the pan, collects in the reservoir at the lowest part. The oil is filtered and then poured into clean glass bottles, in which the impurities are allowed to settle.
Pure oil of lemons is almost colorless and has the odor of the fruit. Its specific gravity varies between 0.8752 and 0.8785; it boils at 298.4° F., and is soluble in all proportions in absolute alcohol and glacial acetic acid. It contains, according to G. Bouchardat and J. Lafont, besides a little cymene, several hydrocarbons, the most abundant of which is citrene, C10H16, boiling near 352.5° F., having a rotatory power exceeding +105°, and yielding a solid optically inactive dihydrochloride.
Limes, oil of (oleum limettæ), is derived from the rind of the fruit of Citrus limetta, or lime. The oil is obtained in the same manner as oil of lemons, which it somewhat resembles. Its mean specific gravity is 0.8734 at 84° F. The oil made by the écuelle process is of a decidedly yellow color, varying in intensity, being darker in the fresh product. The difference in flavor and aroma is so marked as scarcely to require any other means of distinguishing the oil made by the écuelle process from that obtained by distillation, the first having a decided fragrant lemon smell, whilst the distilled oil is very inferior, frequently possessing little more than the smell of turpentine. Large quantities of oil of limes are manufactured in Dominica, but most of the oil exported from there is of an inferior quality and was formerly solely used for adulterating oil of lemons. Lately it has also been employed for scenting soaps and in the manufacture of the common essences and perfumes.
Or. smyrnæum
Myrrh oil (oleum myrthæ) is obtained from the leaves of Myrtus communis, L. It is yellowish, dextrorotatory, of specific gravity 0.910 at 60.8° F., and commences to boil at 320° F. As its principal constituents, Jahns has established a terpene (C10H16), boiling at from 316.4° to 320° F., and turning the plane of polarization to the right, and cineol (C10H16O), which boils at 348.8° F. Besides these principal constituents there seems to be present a very small quantity of a camphor, corresponding very likely to the formula C10H16O. Myrtol, which was formerly supposed to exist in myrtle oil, has been found to be a mixture of the dextrorotatory terpene and of cineol.
Very similar to orange-peel oil, though differing somewhat in odor, is the mandarin oil obtained from the fresh peels of the mandarin orange, the fruit of Citrus sinensis. It is brought into commerce from Reggio and is said to form a constituent of the genuine ess-bouquet. It has a specific gravity of 0.852 at 50° F. and is dextrorotatory.
Oil of orange flowers is at first nearly colorless or straw-yellow, but becomes reddish-yellow when kept for some time. In a clear glass it opalizes bluish. It is limpid and has a slightly bitter taste and a strong but very delicious odor. Its specific gravity varies between 0.85 and 0.90. It is but sparingly soluble in water, but imparts to the latter an agreeable odor; the solution is colored red by sulphuric acid. With 1 to 2 parts of 90 per cent. alcohol, the oil gives a clear solution which becomes turbid by a further addition of alcohol and, after standing quietly, a flaky separation of stearoptene is observed. If not carefully kept the oil becomes darker and even acquires a disagreeable odor. By rectification with water oil thus spoiled can be restored. With sodium the oil does not evolve hydrogen gas; it detonates with iodine.
Neroli oil is much used for the finer perfumes, it being especially a necessary constituent of eau de Cologne. It has to be carefully protected from air and light. For perfuming purposes it is only sufficiently ripe after having been stored for at least one year. If, however, it becomes too old, it inclines towards rancidity, which may be prevented by compounding it with an equal volume of fine spirit. Portugal oil being of inferior quality can only be used for lower grade products.
Oil of orris root serves as a substitute and for strengthening the natural odor of violets. It can, however, be employed only for very fine expensive perfumery since, on account of the very slight yield from the root (from 1000 parts ½ to ¾ part of oil), its price is very high, generally exceeding that of rose oil.
The peculiar, penetrating, though not exactly agreeable, odor of patchouli leaves, is due to a volatile oil, of which they contain 1.5 to 2 per cent. In the Orient this oil has for many years been obtained by distillation.
Peppermint oil (oleum menthæ piperitæ) is obtained from the fresh flowering peppermint, Mentha piperita, natural order Labiatæ. In commerce, German, English, American and Japanese peppermint oils are distinguished. As regards fineness, the German oil is inferior to the English and better kinds of American oils, but superior to the Japanese. The best and most expensive oil is the English so-called "Mitcham oil of peppermint," which dissolves in 50 parts of 50 per cent. spirit of wine and possesses a fine, pure taste, it being for this reason preferred by distillers for the fabrication of liqueurs. For perfuming purposes, however, the American as well as the German oils are very suitable. Of American oils that of H. G. Hotchkiss, L. B. Hotchkiss, Hale & Parshall, and Fritzsche Bros. enjoy a high reputation. The Japanese oil is distinguished from the rest by a peculiar train-oil-like odor and taste, and is in but little demand.
American, German and English oils of peppermint may be distinguished as follows: By adding to 5 or 6 drops of the oil, 25 to 30 drops of pure white concentrated sulphuric acid, American oil of peppermint becomes heated and emits vapors, the mixture acquiring a dark brownish red coloration. After mixing with 8 to 10 cubic centimeters of 90 per cent. alcohol, the fluid becomes turbid, pale yellowish brown, or reddish brown, and on boiling clear pale brown. When mixed with sulphuric acid, German oil of peppermint becomes heated without emitting vapors, becomes yellowish red, not very dark, and turbid. After diluting with alcohol, the fluid becomes turbid and yellowish red, and on boiling somewhat more transparent and currant-red. English oil of peppermint treated in the same manner as the others with sulphuric acid becomes very slightly heated without any emission of vapor. After diluting with alcohol, it becomes clear and raspberry red.
As adulterants of peppermint oil are mentioned: fat oils, alcohol, oil of turpentine, copaiba oil, mustard oil, and ginger oil. The most frequent adulteration is an admixture of oil of turpentine. It is recognized by the oil not dissolving clear in equal parts of 90 per cent. alcohol, as is the case with the pure product. To detect the presence of copaiba oil, mix 5 drops of the oil to be tested with 15 to 20 drops of fuming nitric acid, shake and allow it to stand for 1 to 2 hours. After this time the oily portion should be neither entirely nor partially congealed, but remain fluid. To detect traces of mustard oil, bring 10 drops of the oil into a wide reagent glass, then add 3 to 4 cubic centimeters of absolute alcohol, 2 to 3 drops of silver nitrate solution and 12 to 15 drops of ammonia. The mixture is clear and colorless, and remains so on heating to boiling. In the presence of mustard oil turbidity and blackening take place in consequence of the formation of silver sulphide. After boiling, allow the fluid to stand quietly for 2 or 3 hours. If it then shows a grayish turbidity, the oil is adulterated with another volatile oil.
Poley oil (oleum menthæ pulegii).—In Southern France and Spain this oil is obtained by distillation from the leaves of Mentha pulegium. It has an odor resembling that of peppermint, is at first colorless, but soon becomes yellow, has a specific gravity of 0.927, boils at 361.4° to 370.4° F., and contains neither menthol nor carvol. It is used for perfuming herb soaps.
Rose oil or attar of roses (oleum rosæ) comes almost exclusively from Bulgaria, where it is obtained by distillation with water from the flowers of Rosa damascena. The small quantities of an excellent quality of rose oil prepared in Southern France from Rosa provincialis remain in the country of their production and do not even cover the local demand. The small productions of Persia and India need also not be taken into consideration. In Tunis, where formerly much rose water was prepared from Rosa canina and also rose oil of a very fine quality, the distillation of roses has, according to Christo Christoff, been entirely abandoned, geranium oil only being now produced. In the summer of 1884, Schimmel & Co., of Leipzic, Germany, made the experiment to obtain oil on a large scale from indigenous roses. The result was very satisfactory, 2000 lbs. of rose leaves yielding about 1 lb. of oil, the extraordinarily agreeable odor of which was so superior to that of the Turkish oil, that notwithstanding the high price—double that of Turkish oil—it found ready purchasers. At the ordinary temperature the Leipzic oil is solid, it only melting at 89.6° F.
The flowers are gathered before sunrise, and, if possible, the same day subjected to distillation. The latter is effected in a very crude apparatus, over a direct fire. The flowers are distilled with double their weight of water, one-half of which is drawn off. The product of several operations thus obtained is combined and again distilled, when, however, only one-sixth is drawn off. This distillate is allowed to stand for one or two days in a place warmer than 59° F., when the oil floating on the top is skimmed off. It may be supposed that on an average 6600 lbs. of roses are required to obtain 2.2 lbs. of oil, and that these 6600 lbs. of roses correspond to an area of 1 hectare (2.471 acres) planted with rose bushes.
The larger or smaller content of stearoptene in rose oil seems to be dependent on climatic conditions, it having been remarked that the quantity is the greater the lower the temperature of the region. The oil from the coldest and highest regions of the Balkan is richer in stearoptene than that from the lower and warmer regions.
For the insulation and determination of the stearoptene in rose oil, Schimmel & Co. proceed as follows: Heat 50 grammes of oil together with 500 grammes of 75 per cent. alcohol to from 158° to 176° F. In cooling, the stearoptene separates nearly quantitatively. Separate it from the fluid, treat it again in the same manner with 200 grammes of 75 per cent. alcohol, and repeat the operation until the stearoptene is entirely free from odor. Two treatments of the crude stearoptene are generally sufficient. In this manner Schimmel & Co. obtained from 1887 German rose oil 32½ per cent. stearoptene, from 1888 German rose oil 34 per cent., from 1887 Turkish rose oil 12 to 13 per cent., and from 1888 Turkish rose oil 14 per cent.
Many attempts have been made to fraudulently make this congelation appear within the limits of temperature permitted, paraffine which dissolves well in rose oil being formerly frequently added. In such case the oil may congeal at from 65.75° to 68° F., but the crystals are opaque, dirty yellow, and dissolve to a turbid paste which collects on the surface. The simplest method is to distil white roses with the red. The resulting product has not as fine an odor as that from red roses alone, but is richer in stearoptene. Such oil, which, unadulterated, congeals perhaps at 68° F., can by the addition of geranium oil be reduced to from 63.5° to 65.75° F., thus keeping within the limits permitted.
Schimmel & Co. give a method for an approximate quantitative determination of spermaceti in rose oil: Boil 3 to 5 grammes of stearoptene, separated in the manner above given, with 20 to 25 grammes of 5 per cent. alcoholic potash lye for 5 to 6 hours; then evaporate the alcohol and compound the residue with hot water. In cooling, the greater portion of the stearoptene separates in a crystalline mass upon the surface. Now pour off the alkaline fluid, wash the stearoptene with cold water, then melt it again in hot water, allow it to cool, pour off the water, and repeat the same operations until the wash-water is neutral. The combined aqueous fluids are twice shaken with ether to remove any stearoptene suspended in them. The alcoholic lye separated from the ether is acidulated with dilute sulphuric acid and again extracted with ether. After evaporation no residue (fatty acids) should remain. To control the experiment weigh the regained stearoptene dried at 194° F., adding, of course, the ether used for extracting the alkaline fluid. There will be a small loss, since small quantities of stearoptene always evaporate in drying.
Safrol is very suitable for perfuming ordinary soaps. It has in a still higher degree than camphor oil the property of removing the disagreeable odor of some fats, while at the same time it imparts to the soaps an aromatic, refreshing odor. As a rule 8 to 11 ozs. are used for 220 lbs. of soap; but if it shall at the same time serve for removing the disagreeable odor of low quality fats, especially those extracted with bisulphide of carbon or benzine, it is advisable to take 2.2 lbs., or still better, 4.4 lbs. for 220 lbs. of soap. In this case the safrol should be added to the fat after melting and before saponification and thoroughly mixed with it by stirring. An excellent perfume for ordinary soaps is a mixture of safrol and citronella oil, it being at any rate preferable to oil of mirbane.
The oil distilled from the field thyme, Thymus serpyllum, L., is limpid, yellowish to gold yellow, and of specific gravity 0.89 to 0.91. Old oil is red or brown and no longer limpid. Good oil is soluble in every proportion in 90 per cent. alcohol and emits only slight vapors when brought in contact with iodine. It consists largely of thymene and cymene, and contains a few per cent. of phenol-like bodies.
Venetian oil of turpentine, from Venice turpentine of Larix decidua, Mill., is laevorotatory and resembles the preceding, but has a more agreeable odor. Venice turpentine is mostly obtained in Southern Tyrol and in Piedmont, and yields 18 to 25 per cent. of oil.
Balsam-pine oil (oleum abietis canadensis) is obtained in Canada from the branches of Abies balsamea, D. C. It has a slightly yellowish color, a very agreeable and refreshing odor; specific gravity, 0.902; boiling point at 320° to 330.8° F., and turns polarized light to the right.
Vitivert or vetiver oil (oleum iva ranchusa) from the so-called cuscus, the rhizome of an Indian grass, Anathereum muricatum. The oil is obtained by distillation, either from the fresh root in India, or from the imported dried root in Europe. The yield is very small. The oil is thickly-fluid, of a red-brown color, and has an intense, but agreeable odor very much like that of oil of orris root. Like the latter, it possesses the valuable property of diffusing a lasting perfume. Its value can only be judged by the odor, and hence it should only be purchased from a thoroughly reliable firm.
Wintergreen oil is also obtained by distillation from Gaultheria punctata and Gaultheria leucocarpa. An oil, very closely resembling wintergreen oil, is in this country distilled from the young shoots of the American species of birch, Betula lenta, variously called sweet birch, black birch, cherry birch, and mountain mahogany. According to Procter, the oil does not exist in the birch but is formed by the action of the water upon an odorless body, called gaultherin, which is converted into volatile oil by the reaction of another substance analogous to emulsin. Hence the formation of oil is similar to that of oil of bitter almonds. To obtain the oil from Betula lenta, the material is chopped up and placed in the still, as much as this will hold, a sufficient quantity of water being then added to fill the still about one-third full. The still is generally permitted to remain in this condition over night, a fire is made in the morning and distillation proceeds nicely. The manufacture of birch oil is carried on at quite a large scale by Mr. A. H. Seidle, of Middleport, Schuylkill County, Pa.
Etherification succeeds without difficulty, it being sufficient to heat the mixture for some time and then pour it into water whereby the ether separates as a heavy layer of oil. After washing with water distil in a direct current of steam. The ether thus obtained is as clear as water and, as regards its other properties, does not differ from the naturally occurring oil. This artificial wintergreen oil is now much used for perfuming purposes.
Ylang-Ylang oil (oleum unonæ) is obtained by distilling the flowers of Unona odoratissima, indigenous to the Philippine Islands, the Straits of Malacca, and Indian Archipelago. The oil is colorless to yellowish. Its color and specific gravity, however vary very much, according to the season of the year in which it is prepared, the oil distilled in the cold season being more colorless and limpid than that produced in the warm season. The oil has an exquisite odor, partaking of the jasmine and the lilac, and is used in the manufacture of the finest perfumery. Various kinds are found in commerce, that marked "Sartorius" being preferred.
The resins are generally divided into hard resins, soft resins or balsams, and gum-resins. The hard resins are, at the ordinary temperature, solid, hard, and brittle, can be readily pulverized, and contain little or no volatile oil. The soft resins or balsams are kneadable, and sometimes even semi-fluid; they represent solutions of resins in volatile oils, or a mixture of volatile oil and resin. On exposure to the air they are changed by the volatile oil suffering oxidation, they becoming then more or less hard, and may be converted into actual resins. The gum-resins are mixtures of vegetable gum, resin, and volatile oils, and are obtained by inspissation of the milky juice of several plants. When triturated with water they yield a milky, turbid fluid, and dissolve only partially in alcohol.
Benzoin is exclusively obtained from Styrax benzoïn, Dryand (Benzoïne officinale, Hayne), a tree which grows in Java, Sumatra, and Siam. The bark of the tree is slit to allow a fluid to flow out, which concretes on the trunk in the form of grains, or is collected in vessels in which it congeals and assumes the form of lumps ("tampangs"). Older trees which have been frequently tapped for resin yield a product of a lower quality; the grains ("tears") forming, as a rule, the better varieties. When the benzoin collects in large masses it always shows an amygdaloid structure, the grains ("almonds") of a roundish form, smooth termination, homogeneous structure, and paler color, appearing imbedded in a dark, porous, or resiniform mass.
Benzoin is sparingly soluble in chloroform, only partially so in ether, and completely in alcohol. On mixing the alcoholic solution with water, the resin is separated. Petroleum-ether and benzine withdraw only benzoic acid from the dry, powdered benzoin. All varieties of benzoin dissolve in concentrated sulphuric acid to a beautiful purple colored fluid, from which benzoic acid, if present, is separated in crystals by the gradual addition of water. The establishment of the presence of cinnamic acid is best effected as follows: Boil the sample in milk of lime, filter, and treat the solution with hydrochloric acid. The precipitate thereby separated is thoroughly washed, triturated with potassium permanganate and water, and heated, whereby in the presence of cinnamic acid, oil of bitter almonds is formed from the latter, which is readily recognized by the odor.
Peru balsam (Balsamum Peruvianum) is the produce of the Balsam Coast, San Salvador, Central America, where Sansonate forms the central point of the industry. In the mountain forests, back of the coast, grows the balsam tree (Myroxylon Pereiræ, Klotzch; Toluiferæ Pereiræ, Baillon), natural order, Papilionaceæ. The gaining of balsam commences when the tree is five years old, the collecting time beginning in the dry season in the first days of November. The trunks of the trees are belabored with hammers on four places (according to other statements, on twenty to thirty), so that the bark is detached in strips. After a few days the bark thus loosened is burnt off by means of torches, whereupon a balsamic fluid oozes from the young wood, which is absorbed by pieces of cloth or rags, placed upon the denuded places. When the rags are thoroughly saturated with balsam, they are squeezed out and then thrown into an earthen pot filled with boiling water, whereby the balsam is detached and collects on the bottom of the vessel. By this process the Balsamo de trapó is obtained. By boiling the bark, which falls off, a small quantity of a poorer quality of balsam, called tacuasonte, is obtained, which, it would seem, is frequently added to the better quality. Crude Peruvian balsam is a gray-green to dirty-yellow fluid, of the consistency of syrup. The process of purification in use on the Balsamic Coast is as follows: The crude balsam is brought into large iron vessels, holding from 1300 to 1500 lbs. each, and allowed to clarify by quietly standing from 8 to 14 days, the heavy impurities settling on the bottom, while the light dirt, together with the water, appears as foam on the surface. After 8 to 14 days the balsam is drawn off through a cock, located about 4¾ inches above the bottom of the vessel, into a tinned iron boiler, and boiled over an open fire at a moderate heat for 2 to 3 hours. The foam which forms is skinned off, and boiling continued until no more foam appears.
From the very odoriferous flowers of the balsam tree or, according to others, by expressing the fruits, a white Peruvian balsam is obtained, which is, however, seldom found in commerce. It is of the consistency of honey, pale-yellow, smells of vanilla and melilot, and has an aromatic bitter taste. On standing for some time it deposits crystals of myroxocarpin.
Benzine and petroleum-ether dissolve from the Peru balsam only the nearly colorless cinnamein of which it contains up to 45 per cent. The behavior of Peru balsam towards bisulphide of carbon is very characteristic, 3 parts of it giving, according to Flückiger, a clear solution with 1 part of bisulphide of carbon; if, however, 8 parts more of the latter be added, up to 30 per cent. of a dark resin is separated, while the bisulphide of carbon is but slightly colored.
A content of asphaltum is readily detected by mixing the Peru balsam with ether compounded with about ⅛ alcohol. Any asphaltum present remains undissolved, and may be collected upon a filter.
Tolu balsam is the produce of Myroxylon toluiferum, Humb., Bonpl. et Kunth, Toluifera balsamum, L., a tree of the natural order Papilionaceæ, growing in Northwestern South America. It exudes during the heat of the day, and is collected in gourds. It soon hardens, by which it is distinguished from Peru balsam. In commerce two varieties of Tolu balsam are found, one of the consistency of turpentine and the other solid. The first variety, Brazilian balsam, forms a semi-fluid, turpentine-like, sticky mass, of the color of copaiba balsam. By long storage it becomes hard and brownish. The solid variety, Tolu, or Carthagena balsam, is a brittle, more or less translucent yellow-brown or red-brown resin of a granular or crystalline appearance. It softens at about 86° F., and melts between 140° and 149° F. Viewed under the microscope, it appears rich in crystals of separated ciannamic and benzoic acids. Its specific gravity varies between 1 and 2. Both varieties of Tolu balsam have an aromatic, slightly pungent and sourish taste, resembling somewhat that of Peru balsam. They are readily soluble in ordinary spirit of wine, alcohol, acetone, chloroform, and potash lye, but insoluble in petroleum-ether and bisulphide of carbon. In Tolu balsam have been found toluene, cinnamic and benzoic acids, and several resins not yet sufficiently examined. According to Scharling, toluene constitutes about 1 per cent. of the Tolu balsam. It forms a colorless, limp oil, boils, according to Deville, at 338° F., and according to E. Kopp, at between 309° and 320° F., and has a specific gravity of 0.858. It has a sharp, pungent, pepper-like taste, and an odor resembling that of elemi. In the air, it is gradually converted by oxidation into a soft resin, without, however, becoming colored.
According to Holmes and Nalor, a Tolu balsam differing in its chemical behavior is found in the English wholesale trade. In thick layers it is yellow-brown, but perfectly transparent and gold-yellow in thin layers and extraordinarily sticky. By storage it hardens but slightly, and does not become brittle even if exposed for several days to a temperature of 212° F. Its odor reminds one somewhat of glue, and it develops a pungent, sharp taste only after chewing it for a few seconds. Its melting point lies at 136.4° F., being lower than that of ordinary Tolu balsam, from which it also differs in that it completely dissolves in ether as well as in benzine, while it is only partially dissolved by potash lye. The balsam contains no toluene, nor a hydrocarbon, boiling at 320° F. Further investigations have shown it actually to be a natural product, the derivation of which, however, could not be ascertained.
Liquid storax contains styrol (10 to 15 per cent.), styracin, and cinnamic acid (10 to 15 per cent.). Styrol or cinnamol seems to be the most important carrier of the odor and taste of liquid storax. If 20 parts of liquid storax are subjected to distillation together with 15 parts of crystallized soda and 200 parts of water, the cinnamol collects in the form of a yellowish, very mobile liquid upon the distillate. By rectification it can be obtained colorless, but is thereby partially converted into metastyrol, an isomeric, amorphous, odorless, and tasteless substance which is solid at an ordinary temperature. By exposure for some time to a heat of 608° F. it is reconverted into styrol. Styrol (C8H8) forms a clear, colorless, mobile liquid having an odor of benzine and naphthalene. Its specific gravity is 0.924 and its boiling point lies at 294.8° F. In water it is but sparingly soluble, but is miscible in all proportions with anhydrous spirit of wine, chloroform, benzine, ether, and oils. It stands in the same relation to cinnamic acid as benzol to benzoic acid, and is formed by distilling a mixture of cinnamic acid and barium oxide.
Liquid storax is said to be adulterated with the turpentines of some species of larch and pine. Such adulteration is primarily detected, according to Hager, by the specific gravity. Take up a drop of the balsam with a knitting-needle, and by heating the needle make it fall into a cold solution of 1 part common salt and 8 parts water. On stirring, the drop must sink, otherwise adulteration with turpentine is very likely. Next bring 5 grammes of the storax into a test-tube, melt it in the water-bath, add ½ volume of absolute alcohol, and mix by shaking; then compound the mixture with several times its volume of petroleum-ether, shake vigorously, allow to settle, and decant the layer of petroleum-ether. Repeat twice this shaking with petroleum-ether; then evaporate the petroleum-ether solution in a tared flask in the water-bath. The residue remaining after evaporation is colorless, bluish opalescent, and of an agreeable odor; in the presence of turpentine it is yellowish and has the, not to be mistaken, odor of turpentine.
Ordinary storax (Styrax calamitus or St. vulgaris) is an artificial product prepared by mixing liquid storax with various comminuted vegetable substances. Formerly the above-mentioned bark of the storax tree (Cortex thymiamatis) was only used for this purpose, but at present sawdust and exhausted cinnamon are also taken. This storax forms a reddish or brown-black, humus-like mass, which is generally moist. When dried it is very friable and has a storax-like odor distinctly calling to mind that of cinnamon. Good qualities are abundantly covered with crystalline efflorescences (of cinnamic acid and styracin); poorer qualities prepared with the addition of sawdust have a musty odor. The admixed vegetable tissue can, according to Wiesner, be readily recognized by boiling the storax with alcohol, and after washing treating with dilute chromic acid, to which a small quantity of sulphuric acid has been added.
Balsamodendron Ehrenbergianum, Berg
In commerce Myrrha electa and Myrrha vulgaris or in sortis are distinguished. Myrrha electa, the best quality, occurs in pieces of irregular form and variable sizes, consisting of tears—either distinct or agglomerated—usually covered with a fine powder or dust. The surface is seldom smooth, but generally rough or granular. The color varies, being pale reddish-yellow, red, or reddish-brown. The fracture is conchoidal, seldom smooth, but rather granular, rough, of a fatty lustre, and sometimes shows whitish striæ or veins, or opalesces like flint. The fractured edges are more or less translucent; thin disks or splinters are translucent or transparent. The specific gravity is, according to Hager, 1.195 to 1.205, and according to Ruickholdt, 1.12 to 1.18. A Myrrha electa is the better, the more fragile, friable, and paler in color it is, and the more rapidly it ignites and burns with a yellow, sooty flame. Poorer qualities may be recognized by the dark-brown color and dirty appearance. Myrrh is with difficulty rubbed to a fine powder, this being possible only after drying, which must, however, be done at a very moderate heat in order to prevent loss of volatile oil.
Myrrh is frequently contaminated with bark, which forms either a film of cork as thick as paper or a crust of a fibrous and, at the same time, brittle nature. Sand or small pebbles are also frequently mixed with the myrrh. Other varieties of gum or gum-resin, which considerably decrease the value of the product, are often found in the commercial article, the inferior qualities especially being adulterated and mixed with dark pieces of Suakim gum, gum of the plum or cherry tree, bdellium, and similar substances, which are partially moistened with myrrh tincture, and scattered over with myrrh powder. Adulteration with gum-arabic, gum of the plum or cherry tree, which are coated with alcoholic myrrh solution, is recognized by the paler lustre, greater transparency, and mucilaginous taste. Pieces of resin melt on heating, while myrrh only swells up. Bdellium is detected by the dark or black-brown color, toughness, less bitter taste, and by crackling and spitting when held in the flame of a candle, as well as by the reaction of myrrh with nitric acid discovered by Bonastre. By mixing 5 cubic centimeters of alcoholic myrrh tincture with 5 to 10 drops of fuming nitric acid, a rose-color coloration passing into red results. Parker gives the following method for testing myrrh: Prepare a tincture of 1 part myrrh and 6 parts spirit of wine. Saturate with this tincture white filtering paper, allow it to drain off, and then wrap it around a glass rod moistened with nitric acid of 1.42 specific gravity. With genuine myrrh the paper immediately becomes deep yellow-brown and then black, while the edges of the paper strip appear dark purple-red. When a few drops of the tincture of myrrh are allowed to dry in, a transparent residue remains behind. The tinctures of spurious articles (with the exception of bissabol) give turbid residues.
Olibanum or Frankincense is the inspissated juice of various varieties of Boswellia, partially indigenous to Africa and partially to Asia. The pure pieces are pale yellow, seldom reddish, transparent, or opaque, brittle, covered with a mealy coating and of a splintery fracture. The specific gravity of olibanum is 1.22; its odor is slightly balsamic, and its taste bitter and pungent. It melts only incompletely when exposed to heat, diffusing an agreeable odor. It consists in 100 parts of 5 to 7 parts of a clear volatile oil, boiling at 323.6° F., and of specific gravity 0.86, 56 parts of acid resin, and 30 to 36 parts gum, which corresponds with gum-arabic. With water it forms a milky fluid, and is mostly dissolved by spirit of wine. Selected olibanum (Olibanum electum) is the best commercial variety, while Olibanum naturale, O. in lacrymis, and O. in sortis, form darker pieces intermingled with separate paler grains, and contaminated by pieces of bark, and wood and sand.
Kabardin, Siberian, or Russian musk (Moschus sibirius, or cabardinicus) is a cheaper variety of an inferior quality, which is brought from Mongolia and Siberia. The sacs (Fig. 23) are longish, generally pear-shaped, flatter in proportion to their longitudinal and latitudinal dimensions, and not of a puffed-up appearance, the surface being frequently even shrivelled or wrinkled. The outer skin is denser and harder, and on the convex side covered with longer hair (up to 0.9 inch long), of nearly a silver or brownish color. Towards the edge of the sac the hairs are, however, frequently so trimmed and shorn as to give the sac a resemblance to the Tonkin article. The musk-substance inclosed in the sac amounts to from 8.46 drachms to 1 oz. It is somewhat paler, more brown or yellow-brown, soft, almost unctuous, when fresh, but after storing, solid or granular-pulverulent, like ground, burnt coffee. The odor is weak, offensive, more urinose, resembling that of castor, or horse sweat.
The musk-substance of the Tonkin sacs is generally a heavy, dry-feeling mass; it is partially intermingled with and partially enveloped by small, thin, soft, brown, somewhat transparent membranes and frequently mixed with small hair. It is partially loose and crummy, and partially consists of various lumps or grains of the size of a mustard seed to that of a pea, which are more or less roundish, more seldom angular, softer or harder (but can always be readily cut), of a fatty lustre and black-brown or dark-red color. In fresh sacs, the mass is frequently soft, and, when bruised, somewhat smeary, but never unctuous. On rubbing, it becomes paler in color, and glistening hair-like, paler, gray or whitishyellow particles, sometimes of a crystalline texture, appear. The odor of the musk substance is peculiar, strong, and very constant; it is agreeable only when much diluted.
Since, on account of the high price of musk, the musk animal is much hunted, there is a possibility of it becoming in time extinct. For this reason a substitute has been long searched for, and is believed to have been found, especially, in the American musk-rat (Fiber zibethicus), which is chiefly hunted for its skin. In this animal the musk is found in two small sacs located between the anus and generative organs, and is emitted when the animal becomes excited. According to R. S. Cristiani, this musk is invaluable for the toilet soap industry of America, it being nearly as good and strong as genuine musk. Cristiani has formerly used much of it for scenting soaps, powders, etc., but does not recommend it for essences. When used for soaps, some time is required for the odor to become refined, and if a piece of soap scented with it is stored for a few months, it would, according to Cristiani's assertion, be difficult even for an expert perfumer to distinguish the odor from that of genuine Tonkin musk.
A process for the preparation of artificial musk has been patented by Dr. Baur, of Gispersleben. According to the specification, toluol is mixed with the halogen compounds of butane and boiled with the addition of aluminium chloride or aluminium bromide. The product of the reaction is mixed with water and distilled with steam. The fraction passing over between 338° and 392° F. is caught and treated with fuming nitric acid and fuming sulphuric acid. The product obtained is washed with water and alcohol, and crystallized. The artificial musk forms an amorphous white powder, which in time becomes yellow. It is readily soluble in 90 per cent. alcohol, but from solutions in weaker alcohol it again crystallizes out at a cool temperature. The odor becomes very pronounced after the addition of 5 drops of ammonia to 1 pound of a one per cent. solution.
Musk is very much adulterated, the Chinese being adepts in this sophistication. Dried blood, on account of its resemblance to musk, is among the most common adulterations, but, besides this, sand, iron filings, hair, the dung of birds, wax, asphaltum, and many other substances are introduced. They are mixed with a small portion of musk, the powerful odor of which is communicated to the entire mass, and renders the discovery of the fraud sometimes difficult. The bags containing the musk should have the characteristics before described as belonging to the natural sac, and present no sign of having been opened. One of the grossest frauds, which is also perpetrated in Europe, consists, according to Hager, in perforating the musk sac with a needle, placing it in strong rum or weak spirit of wine, and, after pressing it with the fingers, washing with spirit of wine and drying in the air. By this means a tincture suitable for perfuming purposes is obtained, while the musk-substance is increased in weight by the absorption of moisture. Sacs thus treated are, however, readily recognized, they being, after drying, gnarled and uneven.
Civet (zibethum) is derived from two animals of the genus Viverra. The actual civet cat (Viverra civetta, L.) lives in the hottest parts of Africa from the Guinea Coast and the Senegal to Abyssinia, where it is carefully bred for its civet. The product is also obtained from Viverra zibetha, L., indigenous to the Moluccas and Philippines. The civet is secreted in a cavity between the anus and the external genitals, and is scraped out with a spoon. It is semi-liquid, unctuous, yellowish, becoming brown and thicker by exposure to the air, of a bitter, disagreeable, fatty taste, and of a peculiar, urinose, disagreeable odor, resembling that of musk which becomes agreeable only when much diluted and mixed with other perfumes. When ignited it burns with a bright flame, leaving behind 3 to 4 per cent. of ash. It is insoluble in water; in spirit of wine it partially dissolves with difficulty, and with greater ease in warm ether and in chloroform. It should form a homogeneous, non-crumbling mass. According to M. Boutron Chalard, it contains free ammonia, stearin, olein, mucus, resin, a yellow coloring substance, salts, and a volatile oil, the latter giving the odor to it. In perfumery, civet is chiefly used as an addition to other perfumes in order to strengthen them and make them more constant. It is also employed for perfuming fine leather articles.
In commerce two principal varieties are distinguished: Siberian or Russian and Canadian, English or American castor, the first being the most valuable. The length of a Siberian sac varies between 2.36 and 4.72 inches, the width between 0.98 and 2.55 inches, and the thickness between 0.78 and 1.57 inches; it weighs from 1.76 to 8.81 ozs. One of the sacs is generally somewhat smaller than the other. The exterior skin of the sac is almost smooth and, in a dry state, dark brown; the interior is dirty yellow, intermixed with a dense cellular tissue, which envelops the castor-substance and is grown together with it. In a dried state, the latter is dark brown, without lustre, almost friable, of a very strong, peculiar odor, and a pungent, somewhat bitter, aromatic taste.
Ambergris is a fatty, waxy substance, often found floating on the sea on the coasts of Arabia, Madagascar, Japan, etc. It is also found in the cæcum of the sperm whale (Physetus macrocephalus, Schow), and is supposed by some to be a morbid secretion in the urinary bladder. According to Mr. Beale, it merely consists of the indurated fæces of the animal, perhaps somewhat altered by disease. It has a gray-white color, often with a black streak and a slight agreeable odor, like that of benzoin, which becomes more pronounced on heating. When held for some time in the hand it becomes soft and flexible. It melts at the temperature of boiling water, and, when more strongly heated, volatilizes in the form of a white vapor, leaving but slight traces of ash behind. Its specific gravity is 0.8 to 0.9. It is insoluble in water, sparingly soluble in cold spirit of wine, and more readily so in hot spirit of wine, ether and volatile and fat oils. It is almost completely soluble in absolute alcohol. Though ambergris crumbles readily, it can only with difficulty be converted into coarse powder. With the finger it can be polished like hard soda-soap.
On account of its high price, ambergris is frequently adulterated, the commercial article being often nothing but an artificial mixture of benzoin, olibanum, wax, and flour, with other substances, perfumed with musk. Such adulterations are detected by the appearance, proportions of solubility, nature of the fracture and the content of ash. A small quantity of pure ambergris, exposed to heat, melts without forming bubbles or scum. It is easily punctured with a heated needle, which, when withdrawn, should come out clean and without anything adhering to it, and the characteristic odor of ambergris should be immediately evolved. The surface should be rugged, that with a smooth and uniform surface being generally factitious.
In speaking of the volatile oils used in perfumery, two artificial perfume-materials, artificial oils of bitter almonds and wintergreen have already been mentioned. There can be no doubt that when the chemical construction of volatile oils is better known, chemistry will succeed in preparing still more such combinations, valuable for perfumery, or in converting cheap volatile oils into more valuable ones, as has, for instance, been done by Bouchardat and Lafont, who have successfully converted oil of turpentine into oil of lemons. These chemists rectified French oil of turpentine at exactly 311° to 314.6° F., dissolved in the distillate, which amounted to 120 grammes, an equal quantity (120 grammes) of glacial acetic acid, cooled the mixture and then carefully added, so that the temperature never exceeded 104° F., 88 grammes of crystallized chromic acid dissolved in a sufficient quantity of acetic acid. Notwithstanding that the greater portion of the oil of turpentine remained unoxidized, a thorough reaction took place, and the product of decomposition proved to be a hydrocarbon, boiling at from 345.2° to 352.4° F., to which Bouchardat and Lafont have applied the term "terpilene." The properties of this hydrocarbon, especially its boiling point, corresponded with those of oil of lemons, its odor also resembling that of the latter, but it contained about one-sixth cymol which it was impossible to remove. Though thus far this artificial oil of lemons is of no importance for perfumery, it is of interest as showing the possibility of converting one volatile oil into another.
Cumarin forms small, colorless crystals of a silky lustre. It is very hard, cracks between the teeth, shows a smooth fracture, and sinks in water. It has a very agreeable aromatic odor, which, on rubbing the substance with the fingers, becomes like that of oil of bitter almonds, and has a bitter, warm, and pungent taste. When pure it melts at 152.6° F., but when containing fat, like that separated from tonka beans, at from 104° to 122° F. Its boiling point lies at 554° F.; it volatilizes, however, at far lower temperatures, diffusing an odor resembling that of oil of bitter almonds, and sublimating in white needles. It is soluble in alcohol, ether, acetic acid, fat, and volatile oils. Of cold water (59° F.) 400 parts are, according to Buchner, required for its solution, but of boiling water only 45 parts.
The Dutch tonka bean is 1.18 to 1.57 inches long, 0.39 to O.59 inch wide, and O.27 to O.43 inch thick. It is generally slightly curved, provided under the point with the hilum, and covered with a thin, fragile, brown-black or black skin of a fatty lustre, upon which small crystals of cumarin are generally found, so that it appears coated, especially in the wrinkles, with a whitish dust. The kernel consists of two yellow-brownish oleiferous catyledons, between which layers of cumarin are generally found. The odor is agreeable, resembling that of melilot, and the taste aromatic bitter. Dutch tonka beans contain fat, sugar, malic acid, and malate of lime; further, starch, gum, and 1 to 5 per cent. of cumarin (C9H6O2). The English tonka beans are smaller, white-yellowish inside, nearly black outside, and of inferior quality to the Dutch beans.
Perkin has recently succeeded in artificially preparing cumarin from salicylic acid. By boiling the sodium salt of the latter in acetic anhydride for a few minutes and then pouring into water, an oil-like body is separated, whilst sodium acetate passes into solution. The former is a mixture of acetic anhydride, salicylic acid and cumarin; in distilling, the latter passes over last (at 554° F.), and congeals in the receiver to a crystalline mass.
Exposed to the action of heat and air, heliotropin acquires an uncomely appearance, balls together and, under very unfavorable circumstances, turns brown. It is then entirely decomposed and useless, and, hence, should be kept in summer in as cool a place as possible. A temperature of 95° F. has already an injurious effect upon the perfume, and it is best not to buy it at all in the hot summer months. To preserve the perfume in its entire freshness, it is advisable for consumers in hot climates to at once dissolve the heliotropin in alcohol and to keep the solution in a cool place.
To obtain piperonal from the potassium piperate, dissolve 1 part of the latter in 40 to 50 parts of hot water, and then slowly introduce, with constant stirring, a solution of 2 parts potassium permanganate in 50 parts of water. This precaution is absolutely necessary, as otherwise the piperonal formed would be partially further oxidized and lost. The paste-like mass formed is passed, while still hot, through a straining cloth, and the residue repeatedly washed with boiling water until it shows nothing more of the characteristic odor of héliotrope. The wash-waters are combined with the first filtrate, and subjected to distillation over a free fire.
Vanillin.—Vanilla is the not entirely ripe, pod-like, capsular fruit (wrongly called pod), of a tropical orchid (Vanilla planifolia, Andrews), which is cultivated in Mexico, the West Indies, and South America. It is extensively used for flavoring, and its odoriferous substance is highly valued in perfumery. The cross-section of the capsule is thick and fleshy, filled with very small, black, lustrous seeds stuck together by a gummy balsam with which they are coated. The capsule has a sourish taste and has no value, the seeds, or rather the balsam enveloping the seeds, being the substance on which the odor and taste of vanilla depend. When the vanilla fruit becomes ripe, the capsule opens and empties its content of seeds in the form of a balsam-like mass.
At present, vanillin is prepared artificially. Tiemann and Harmann first showed that by the oxidation of coniferin, a glucoside occurring in the cambial sap of the Coniferæ, a product, perfectly identical with the vanillin prepared from vanilla, is obtained. The coniferin is obtained by barking the pine or silver fir, scraping together the sap under the bark together with a portion of the liber and pouring it into a vessel. The sap is then pressed off, boiled to separate the albumin, filtered, evaporated to one-fifth its volume, and set aside to crystallize. One hundred quarts of sap are said to yield from 1 to 2 pounds of coniferin-crystals. By now allowing an aqueous coniferin-solution to run into a heated mixture of 10 parts potassium bichromate, 15 parts concentrated sulphuric acid, and 80 parts water, and heating for 3 hours in a flask with back-flow cooler, a liquid is obtained from which ether takes up a yellow oil. After treating the latter with animal charcoal, dissolving in ether and evaporating the latter, there remain colorless, acicular crystals of the odor and taste of vanilla. These crystals consist of vanillin contaminated with some vanillic acid. To separate the latter, purify with acid sodium sulphite and recrystallize. After this operation, vanillin represents a nearly white crystalline powder which melts at from 176° to 177.8° F. In this form it is brought into commerce as a complete substitute for vanilla, 5.64 drachms of it corresponding to about 1 pound of vanilla. A medium-sized pine tree is said to yield vanillin of the value of 80 marks ($19.20).
Vanillin (C8H8O3) forms small colorless prisms of a strong vanilla odor, a warm, vanilla taste, and an acid reaction. It is readily soluble in hot water, alcohol, ether, chloroform, fat and volatile oils, as well as in solutions of caustic alkalies and alkaline carbonates. It melts when heated to from 176° to 177.8° F.; at a higher temperature it sublimates without leaving a residue.
Nitrobenzol is obtained by treating benzol, or a mixture of it, with toluol and their higher homologues, with strong nitric acid, or a mixture of nitric and sulphuric acids, washing the product of reaction with water and soda, caustic soda or ammonia, expelling the unaltered hydrocarbons with steam and rectifying the residue. Three varieties distinguished by their boiling points and odor occur in commerce. The nitrobenzol or oil of mirbane (essence de mirbane) is the so-called light nitrobenzol, which boils at from 401° to 415° F. The heavier varieties boil at a higher temperature and have a more or less disagreeable odor; they are used in the manufacture of aniline and aniline colors.
According to Grossschopf, 40 lbs. of pulverized anhydrous sodium acetate, together with a cooled mixture of 46 lbs. of concentrated sulphuric acid and 37 lbs. of 95 per cent. alcohol, free from fusel oil, are distilled in a copper still heated by steam. Distillation is continued with constant stirring by means of an apparatus in the still, until no more fluid smelling and tasting of acetic ether passes over. The crude distillate, amounting to 55 or 56 lbs., is brought into bottles which are filled ⅔ full. The bottles are then filled up with water and potassium carbonate is added until the fluid, after shaking, shows no acid reaction. The aqueous fluid beneath the ether is then drawn off by means of a siphon, and the ether several times washed by shaking with water and allowing to settle. Since the wash-water absorbs a quite considerable quantity of ether, it is collected and subjected to rectification, whereby an alcoholic acetic ether is obtained. The ether, being freed from acetic acid and alcohol by neutralization and washing, is brought in contact with fused calcium chloride to free it from water, and finally rectified over magnesia. In this manner 36 to 37 lbs. of pure acetic ether are obtained.
Benzoic ether or ethyl benzoate, C7H5O.OC2H5, is most readily prepared by mixing 4 parts of alcohol, 2 parts of crystallized benzoic acid, and 1 part fuming hydrochloric acid, and for some time heating the mixture in a flask. The benzoic acid is thereby gradually and completely converted into ether. The fluid is mixed with water, whereby the ether is completely separated. It is several times washed with carbonate of soda solution, and, for the purpose of withdrawing the last trace of free acid, distilled over lead oxide. It forms a colorless oil of an aromatic odor, specific gravity 1.0502, and boils at 412° F. In cold water it is insoluble. However, like all varieties of ether, it dissolves readily in alcohol and ether.
Butyric acid fermentation proceeds more rapidly by using, instead of rotten cheese, putrefying meat, and in place of sugar, starch paste or mashed boiled potatoes, 1 part of meat to 4 parts of starch or a corresponding quantity of potatoes being employed. The same products are formed as in the preceding process, but much more rapidly, fermentation being finished, according to Schubert, in 5 to 6 days.
A suitable material for the preparation of butyric ether is also the St. John's bread or carob, the pods of Silequa dulcis. Redtenbacher established in them the occurrence of about 2 per cent. butyric acid, which Gruenzweig later on proved to be isobutyric acid. Besides butyric acid and other volatile acids, St. John's bread contains about 40 per cent. of fermentable varieties of sugar, which can be utilized after their conversion to butyric acid. For this purpose Stinde has proposed the following process: Convert the pods together with the seeds to a coarse powder; bring 100 lbs. of this powder into a capacious barrel placed in a warm place, and pour sufficient water of 82.5° F. over it, to form a thin paste; after 4 to 5 days add 24 lbs. of whiting and await fermentation. The paste, which gradually becomes thicker, is from time to time stirred, and, if necessary, a small quantity of lukewarm water added. In summer fermentation is finished in six weeks, after which the preparation of the ether is proceeded with.
The first pound of the distillate is caught by itself, and, after changing the receiver, distillation is continued until but little passes over, even with an increased admission of steam. Thus an abundant yield of alcoholic butyric ether is obtained. When distillation is finished 20 lbs. more of alcohol may be brought into the still; the distillate obtained thereby being still rich in butyric ether.
The glycerine does not undergo alteration thereby. The nascent formic acid converts the alcohol present into formic ether, water being separated. When, after continued heating, the development of carbonic acid abates, add the same quantities of oxalic acid and alcohol to the contents of the still, heat again until but little carbonic acid is evolved, and then add, twice in succession, the same quantities of oxalic acid and alcohol as before, until finally as much oxalic acid is consumed as glycerin has been employed. When the evolution of carbonic acid ceases, the receiver is reversed and the ether distilled off. The glycerin remaining behind is again concentrated to the consistency of syrup, and may be re-used.
Nitrous ether, or ethyl nitrite, C2H5.ONO.—In a pure state this ether is best prepared according to the method given by E. Kopp. It consists in bringing equal volumes of alcohol and ordinary nitric acid together with copper filings into a distilling apparatus, which is so arranged that the vapors first pass through a flask filled with water of 77° F., then through a calcium chloride tube, and are finally condensed in a receiver surrounded by snow and common salt. The nitric acid is first decomposed by the copper, nitrous acid being thereby developed, which is so transposed that its radicle NO occupies the position of the typical hydrogen in the alcohol, while the rest of the acid forms water with the hydrogen of the alcohol. By the reaction such a quantity of heat is liberated that the process requires assistance by external heating only towards the end of the operation. In the receiver is then a pale yellow fluid having the taste and odor of apples and, at 59° F., a specific gravity of 0.947. According to Liebig, the boiling point of nitrous ether lies at 61.5° F.; hence it can be condensed only by careful cooling, and has to be kept in glass tubes fused together. In water it is but sparingly soluble, but readily so in alcohol. By the addition of water it is separated from the alcoholic solution.
Everything being prepared, but little steam is at first introduced into the iron cylinder in order to slowly warm the apparatus. When this is done the admission of steam is gradually increased. The mats or pack-cloth placed between the walls of the cylinder and the flask prevent the latter from bursting, which otherwise might readily happen. Distillation commences in about ten minutes. The admission of steam is then moderated, care being had that the ether passes over in an uninterrupted stream of the thickness of a goose-quill.
The ether thus obtained is a fluid, which, in a concentrated state, does not possess an agreeable odor, but when mixed with 10 parts of alcohol imparts to the latter an odor resembling that of apples. It boils at from 370° to 374° F., and at 64° F. has a specific gravity of 0.8793.
Apricot essence.—Chloroform 10, butyric ether 100, valeric ether 50, peach oil 10, amyl alcohol 20, butyric amyl ether 10, tartaric acid[11] 10, glycerin 40.
The method of preparing the flower-pomades in France has already been described on p. 58 et seq. It need here only be added that, according to their quality, these pomades are designated by different numbers by the French manufacturers. There are three qualities, which by some manufacturers are designated as No. 6, No. 18, and No. 30; and by others as No. 12, No. 24 and No. 36, so that No. 6 and No. 12, No. 18 and No. 24, as well as No. 30 and 36 correspond to each other. Pomades No. 6 or No. 12 are not suitable for the preparation of extracts, they containing but little actual extract of flowers, and are generally mixtures touched up with volatile oils. They are almost exclusively used for hair pomades, for which they are well adapted. No. 18 or No. 24 is the quality generally employed by the perfumer for alcoholic extracts. No. 30 or No. 36 is the strongest, and, hence, most expensive flower-pomade, and is used only by a few perfumers who have customers for the finest qualities of Extraits d'Odeurs.
In order to show how the extraction of flower-pomades is effected, we will take, as an example, 2 lbs. of French flower-pomade No. 18 and 3½ quarts of best alcohol.[14] This proportion yields a good and sufficiently strong extract for the preparation of Extraits d'Odeurs. It must, of course, be suited to the size of the extracting apparatus, 8 lbs. of flower-pomade and 14 quarts of alcohol being, for instance, taken, though that depends on the quantity of the respective extract required by the perfumer. It is, however, best that the apparatus should be as completely filled as possible so that it contains but little air.
Where the manufacturer has steam-power at his disposal, the apparatus may be connected with the transmission and allowed to run for 48 to 60 hours during working time. After the expiration of this time, proceed to strain off the finished extract (No. 1) as follows: Over a clean tin vessel stretch a close, white linen cloth, and pour the entire contents of the apparatus upon the latter; the liquid portion runs through the cloth into the vessel, while the pomade remains behind upon the cloth. Finally, the cloth is thoroughly wrung out in order to obtain as much alcoholic extract from the pomade as possible. Bring the extract, No. 1, thus obtained into a glass flask, allow it to stand in a cool cellar for about 48 hours, and then filter it through paper into another glass bottle. This filtering through paper is necessary, even if the extract should appear clear and pure, as, in straining, not only do small particles of fat pass through the cloth, but are also dissolved in the extract. By quietly standing in a cool cellar these particles of fat are separated and appear as white flakes on the bottom and sides of the flask. At a higher temperature, these flakes melt and appear as drops of oil on the bottom of the flask. If filtering were omitted, these particles of fat would be transferred to the extracts and thus cause stains upon handkerchiefs, clothing, etc. If the manufacturer has not a cool cellar at his disposal, the fatty particles are readily separated by placing the flasks containing the extract upon ice, and filtering immediately after separation is complete. The fat then remains upon the filter.
Beyer frères, of Paris, have essentially improved the extracting apparatus previously described, the improvement being shown in Fig. 26. The cylinders A and A1 are of copper tinned inside; the lids close air-tight; above the cocks f and f1 a perforated piece of tin is placed in the interior of the cylinders; upon this piece of tin a disk of felt may be placed, and thus the extract be drawn off clear. In order to reduce the pomade to a finely divided state, and thus bring it in contact with the alcohol, it is passed through a vermicelli press, h, placed upon the cylinder A1. The pomade passes, in the form of fine vermicelli, through a sieve in the lower portion of the press into the alcohol contained in the cylinders. The press can be transferred from one extracting vessel to the other. The shafts a and a1 also have several horizontal arms like those shown in Fig. 25. Through the contrivances d and d1, sitting upon the shaft c, the shafts a and a1, receive a revolving as well as an up-and-down motion, so that a complete mixture of pomade and alcohol is effected. By this arrangement the pomade completely yields its perfume to the alcohol in one day, and independent of the quicker work, it has the further advantage that the extracts are of better quality in consequence of not remaining for so long a time in contact with the fat.
The substances to be used for tinctures should be fresh and genuine, and the alcohol free from fusel oil, since a perfect tincture can only be obtained under these conditions. For the preparation of tinctures Beyer frères have constructed very suitable apparatuses (Figs. 27 and 28). By the vigorous and uninterrupted agitation produced by means of such an apparatus extraction is effected much more rapidly and more completely than by treating the substances to be extracted in ordinary bottles and by shaking with the hand.
The receipts given in the following pages have been practically tested and can be recommended as perfectly reliable.
Bring the alcohol into a bottle. Tolu balsam cannot be reduced to a powder, hence it is necessary to keep it right cool, whereby it becomes brittle so that it can be cut up with a sharp instrument and a hammer. The pieces detached are rapidly brought into the alcohol, solution taking place in about 14 days. If the alcohol were added to the tolu balsam, the latter would ball together, rendering solution very difficult. Frequent vigorous shaking is necessary.
Abelmosk grains are the seeds of a plant (Abelmoschus moschatus Mönch; Hebiscus abelmoschus, L.) indigenous to Central Africa, Arabia, and India. They are reddishgray, kidney-shaped, slightly corrugated on the surface, and of an agreeable musk-like odor. The substance producing the musk odor lies in the seed coat. The odor becomes very pronounced on rubbing the seeds between the hands.
The tonka bean is of great importance for perfumery. The tincture prepared from it has an agreeable, penetrating odor, and in mixing it with other odors, great care has to be exercised, so that the tonka odor is not too prominent. The tincture is prepared as follows: Bring the beans, without comminuting them or removing the white coating adhering to them, into a flask, add the alcohol, and let the whole macerate, with frequent shaking, for about 14 days. Then filter off the fluid. The tincture prepared in this manner only contains the cumarin found as a white coating upon the beans, and is used only for the finest products. Now take the beans from the flask, comminute them, return them to the flask, and add 1¼ quarts of alcohol. This extract gives an excellent tincture suitable for products of medium quality.
From many of the above-mentioned perfume-substances, which serve for the preparation of tinctures and are not entirely soluble in alcohol, but leave a residue after extraction, a second infusion may be made. Musk, castor, and the resins dissolve completely, there remaining behind only the impurities and any mineral constituents present which possess no aroma. But all residues from woods, fruits, etc., are suitable for a second extraction, most of the tinctures thus obtained being quite aromatic, and, as will be seen later on in giving receipts, can be very advantageously utilized. For the second extraction less alcohol has to be taken than for the first.
Bring about 5½ ozs. of pulverized sugar into a capacious porcelain mortar, add the rose oil and mix intimately with the pestle. Then pour the thickly-fluid mass through a glass funnel into a glass flask and rinse the mortar with alcohol until the prescribed 6½ quarts of the latter have been brought into the flask. Frequent shaking accelerates the complete solution of the rose oil.
Extrait jacinthe.—Extracts No. 1 from Pomm. Jacinthe 750 drachms, and from Pomm. Acacia 100; bergamot oil 5, clove oil 1, storax tincture 2½, musk-root tincture 12½, tinctures of musk and ambergris 1½ each.
Extrait de violette de Parme.—Extract No. 1 from Pomm. Violette 750 drachms, orris-root oil and bergamot oil each 2½, tinctures of musk, ambergris, and bitter-almond oil each 1½.
Extrait ess-bouquet.—Extracts No. 1 from Pomm. Acacia and Pomm. Cassie each 100 drachms, from Pomm. Jasmin 325, from Pomm. Rose 75, and from Pomm. Orange 250; bergamot oil 40, Ceylon cinnamon oil and clove oil each 5, French rose-geranium oil 10, sandal-wood oil 2½, licari oil 8, rose-oil tincture from Turkish rose oil 75, orris-root tincture 50, tinctures of ambergris and civet each 10, musk tincture 15, musk-root tincture 37½, benzoin tincture 15.
Pomm. Héliotrope
Extrait chypre.—Extracts No. 1 from Pomm. Orange 60 drachms, Pomm. Jasmin 40, Pomm. Cassie 110, Pomm. Héliotrope 40; French rose geranium oil 6, bergamot oil 2, cedar oil 3/5, benzoin tincture 4, orris-root tincture 30, musk tincture 5, civet tincture 4, abelmosk tincture 10.
For the preparation of these stronger products, the employment of a stronger foundation, i. e., of more highly saturated extracts from French flower pomades, is required. For this purpose the French perfumers prepare, under No. 30, flower pomades of all odors which are exclusively used for concentrated Extraits. They are, of course, correspondingly higher in price than those prepared from No. 18, which have previously been treated of.
Extraits d'Odeurs, Quality II.—In addition to the fine extracts given in the preceding section, a small selection of quite cheap receipts for quality II of such extracts is here given, the extracts No. 2 offering sufficient material for their preparation. In the introduction to the previous section, attention has been called to the fact that quite useful tinctures may be prepared from substances leaving behind solid residues, there being also on hand the second extract from the flower pomades.
Extrait ylang-ylang II.—Extracts No. 2 from Pomm. Jasmin, Pomm. Jonquille, Pomm. Orange, and Pomm. Acacia each 250 drachms; bergamot oil 3½, angelica oil 1¼, ylang-ylang tincture 250 diluted with the equal quantity of alcohol, abelmosk No. 2, 25, tonka-bean extract No. 2, 7½, musk tincture No. 2, 4, ambergris tincture 1¼ diluted with the same quantity of alcohol.
Extrait ixora II.—Extracts No. 2 from Pomm. Tubereuse 125 drachms, from Pomm. Cassie and Pomm. Réséda each 175; bergamot oil 2½, orris-root tincture No. 2, 125, musk tincture No. 2, 10, benzoin tincture 12½ diluted with alcohol 12½.
For the preparation of actually good Cologne water employment of the best materials is the first condition. The alcohol must be pure, i. e., free from fusel oil, and 95 to 96 per cent. strong, so as to effect a ready and complete solution of the volatile oils. The latter also should be of the best quality and proper age, i. e., neither too young nor too old. If too young or too recently distilled, the aroma is not thoroughly developed, and if, on the other hand, too old, they have lost the greater portion of their aroma, are thickly fluid, acquire a dark coloration, and are finally converted into a resinous substance in which condition they are entirely unfit for finer products. A cool and dry cellar is required for storing volatile oils, and they must also be protected from air and sunlight.
Cologne water, quality I.—Bring into a large glass balloon 95 to 96 per cent. alcohol of the best quality 7.9 gallons, lemon oil 14.11 ozs., bergamot oil 15, neroli oil 4.23, French extra lavender oil 1.05 oz., rosemary oil 0.7, best German balm oil 0.42; mix thoroughly, and after 14 days add best orange-blossom water and rose water each 2.64 quarts. Mix again thoroughly, and then let stand until wanted for use.
Cologne water, quality V.—Bring into a glass balloon alcohol free from fusel oil 7.9 gallons, Portugal oil 0.88 oz., rosemary oil 0.88, lavender oil, bergamot oil, and lemon oil each 1.76. After standing for 14 days, add 7.9 quarts of distilled water. Proceed in the same manner as given for quality IV.
A selection of receipts for such powders is here given. Their manufacture is not difficult; however, the weighing off of the constituents should be conscientiously done, and in mixing the powders with the volatile oils, etc., care should be had not to cause any unnecessary dust and consequent loss of powder. After mixing, the powder is passed through a sieve.
Réséda sachet powder.—Ground orris root 100 drachms, ground rose leaves 50, ground rose wood 25, clove oil, African geranium oil, and bergamot oil each 2½, musk-root tincture 10, vanilla tincture 5, musk tincture 1, extrait réséda 25.
The mode of fumigating has also to be taken into consideration. It is, for instance, entirely incorrect to place the fumigating agent upon very hot iron, a hot stovepipe, or glowing coals, because in evaporating upon hot iron, it leaves behind an empyreumatic, pungent odor excitatory to cough, while the actual aroma is lost and thus the object of fumigating is frustrated.
The dry fumigating agents, such as powders, pastilles, paper, and lacquer, are less popular than the fluid, it being necessary for the purpose of fumigating to place them upon hot articles, heat them, or burn them. These manipulations develop more or less smoke, which frequently exerts a disagreeable effect upon the respiratory organs. The most injurious of these methods of hot fumigation is that by means of hot coals, whereby the aroma of the fumigating agent is largely destroyed, and the very injurious gas emanating from the coals is inhaled. A heated piece of sheet-iron is, however, very suitable for fumigation by means of powder or lacquer. Scatter the powder upon it or coat it with the lacquer.
Fumigating balsam.—Alcohol 3 quarts, orris-root tincture 1 quart, tinctures of benzoin, tolu balsam, and storax each 17½ ozs., olibanum tincture 8¾ ozs., tinctures of abelmosk and musk-root each 3½ ozs., vanilla tincture 1¾ ozs., Peru balsam 4¼ ozs., bergamot oil 1¾ ozs., lemon oil 14 drachms, African rose geranium oil 11¼ drachms, clove oil and cassia oil each 14 drachms, petit-grain oil 11¼ drachms, fine lavender oil 1¾ ozs.
Ordinary fumigating powder.—Lavender flowers, marigold flowers, corn flowers, rose leaves, rasped orris root each 2 lbs., cloves and cinnamon each 3½ ozs., rasped sanders wood 17½ ozs., rasped cedar wood 8¾ ozs., fumigating balsam 17½ ozs., bergamot oil and African rose-geranium oil each 1¾ ozs., lavender oil 11¼ drachms.
New-mown hay fumigating powder.—Lavender flowers 2 lbs., rose-leaves, rasped sanders wood, and rasped orris root each 1 lb., powdered benzoin, Roman camomile, curled mint and balm each ½ lb., exhausted tonka beans 1 lb., patchouli leaves and bergamot oil each 11¼ drachms, African rose-geranium oil 8¼ drachms, tonka-bean extract and abelmosk tincture each 1¾ ozs., extract from French réséda pomade 3½ ozs.
The saltpetre given in the receipts is dissolved by itself in distilled water and last of all added to the mass. Its object is to keep the pastilles burning after ignition.
Musk fumigating pastilles.—Pulverized genuine linden charcoal 2 lbs., pulverized musk root and orris root each 1 lb., pulverized sanders wood, Siam benzoin, and abelmosk each ½ lb., saltpetre 4¼ ozs., dissolved in distilled water; Tonkin musk 1½ drachms, triturated in distilled water; African-rose geranium oil, Portugal and cedar oil each 5½ drachms. Mucilage of gum-tragacanth as much as required.
In accordance with recent medical directions and opinions soap is again employed, and justly so, for the better cleansing of the teeth, whilst formerly it was generally considered injurious. However, though soap is innocuous to the teeth, it should be used in very limited quantities, since its introduction into the mouth is repugnant to many persons, producing in many cases vomiting. The quality of the soap must also be taken into consideration, and only the best neutral soap in the form of a powder, such as is used for fine milled soaps, should be employed.
Glycerin, which occurs in several receipts for dentifrices and mouth-waters, fulfills a double object; on the one hand, its action upon the teeth and mouth is beneficial, and, on the other, it covers the naturally bitter taste of many substances contained in the preparations, and thus makes them more agreeable to use.
Eau dentifrice Orientale.—Alcohol of best quality 5 quarts, peppermint oil and rose-geranium oil each 1¼ ozs., clove oil 11¼ drachms, extrait rose and ratany tincture each 3½ ozs., vanilla tincture 1¾ ozs. Proceed as above and color rose color with corallin tincture.
A few remarks may here be made regarding the use of tooth tinctures. The tinctures should not be used undiluted, they being apt to make tender gums sore, cause pain, and may even produce inflammation. It is best to dilute the tinctures somewhat with water whereby they become milder and more agreeable to the gums. An excellent article for rinsing out the mouth is obtained by pouring a teaspoonful of the tincture into a tumbler of water.
This paste is prepared in the same manner as No. 1, only the proportion of water has to be taken into consideration. To prevent the mass from becoming too soft, the water should be very gradually added.
When everything is prepared, quickly pour the hot water upon the powder in the enamelled vessel and stir rapidly and thoroughly during the effervescence which immediately takes place. The effervescence gradually ceases and the result will be a beautiful crimson colored mass, the hot water having immediately and completely dissolved the coloring matter of the cochineal. Now, bring the mass into a shallow box lined with clean white paper and place it to dry in an airy room, but do not expose it to the air or sunlight. The next day the mass in the box is thoroughly worked through, this operation being repeated every day until the mass is dry. It is then again powdered, whereby it acquires a rose color, and is then sifted. The powder is perfumed with peppermint oil 1 oz. and clove oil and cassia oil each 5½ ozs., sufficient glycerin to prevent dust, being at the same time rubbed in. With the addition of the glycerin the beautiful crimson color of the powder reappears. This tooth-powder possesses excellent cleansing qualities and can be recommended chiefly to persons having yellow teeth, as well as to smokers whose teeth commence to get black.
Saponification will be slowly effected from the sides of the kettle in about one hour, the mass in the kettle rising somewhat. This rising indicates that the process of saponification is going on. The mass is now again stirred, which must be done carefully and not hastily, as otherwise the soap readily becomes spumous. When the soap again lies quietly in the kettle, it will have the appearance of a white prime grain-soap. Now add the coloring substances and the precipitated carbonate of calcium and thoroughly stir, so that the mass acquires a uniform brown color. Then remove the kettle from the water-bath, add the perfumes with constant stirring, bring the finished tooth soap into the frame, lightly cover the latter with the wooden lid and let stand over night. The next day the tooth soap may be cut up into suitable pieces, which are allowed to dry for about 12 hours and then packed in tinfoil, etc.
Some fats enjoy a special reputation as hair pomades, the property of strengthening the scalp and promoting the growth of the hair being ascribed to them. This is especially the case as regards beef marrow and horse fat,[22] whilst in olden times the bone marrow of the deer (cerval medullæ) and bear's grease were believed to possess this property. Cleopatra is said to have used the latter, and many ladies are at the present time under the impression that they apply it to their hair when they use Pommade à la graisse d'ours. Thoroughly purified lard renders no doubt the same service as the above-mentioned fats.
The purification of the fat, which generally consists of 2 to 3 parts lard and 1 part beef-tallow, is frequently effected as follows: Boil for about one hour 125 lbs. of fat with about 30 gallons of water containing 1 lb. each of common salt and alum in solution, constantly removing the scum formed. After standing for several hours, the fat thus purified is carefully taken off from the sediment and water; it is then, together with 4 to 6 lbs. of pulverized benzoin, for some time heated at 167° F., and finally strained into stone jars, which, after the fat is cold, are closed with a piece of bladder or waxed paper and kept for use. Fat thus prepared keeps for years.
The maceration or extraction of the flowers is effected as follows: The fat, generally consisting of 3 parts lard and 1 part beef-tallow, is melted in an enamelled vessel over the steam or water-bath. The flowers in a clean linen bag are suspended in the fat, and after covering the vessel the fat is kept, according to the strength of the perfume of the respective variety of flower, for a day or two more at a temperature of from 133° to 145° F. The extracted and exhausted flowers are then taken out, slightly pressed out, and thrown away. The same operation with always the same quantities of fresh flowers is then repeated ten to twelve times with the same fat, until it is sufficiently perfumed. The pomade thus obtained, to which some white vaseline is frequently added, is then stirred until cold.
Melt together the vaseline and paraffin, add the Peruvian bark extract previously rubbed up with as little water as possible, and stir in the tannin dissolved in the volatile oils.
Lanolin pomade.—Benzoated fat 4 lbs., benzoinized olive oil and lanolin each 2 lbs., bergamot oil 3½ ozs., cinnamon oil 7¼ drachms, clove oil 5½ drachms, lavender oil 3¾ drachms, nerolin 1 drachm dissolved in a portion of the fat heated to 111° F. Color red with alkannin.
Princess pomade.—Fresh lard 8 lbs., cocoa butter and wax each 1 lb., bergamot oil 3½ ozs., lemon oil and lavender oil each 14 drachms, neroli oil 6¾ drachms. Color rose color with alkannin.
Tonka pomade.—Lard 7½ lbs., spermaceti ½ lb., cumarin 4½ drachms, dissolved in a small portion of the warm fat.
Vaseline Pomades.—Vaseline pomades consisting neither of an animal nor of a vegetable fat, but almost entirely of a mineral fat, form a special division of pomades. On account of its good properties and cheapness, vaseline, which is obtained from petroleum residues, etc., has for several years past been much used in the preparation of pomades. The pomades prepared from vaseline are not only very suitable for oiling the hair, as they never become rancid, but may also advantageously be used as a remedy for chapped skin, inflammation, cuts, burns, etc. For pomades odorless vaseline has to be used, 1 lb. of it requiring about 5½ to 8½ drachms of perfume. Lederin, which has been previously mentioned, is best suited for coloring the pomades. When used for pomades vaseline, though by itself sufficient for the purpose, frequently receives an addition of paraffin, wax (mostly ceresin), and lard. To vaseline pomades intended for export to warm climates, an addition of ¼ to ⅛ceresin is required.
Victoria vaseline pomade.—White vaseline 5 lbs., paraffin 1 lb., bergamot oil 1½ ozs., rose-geranium oil 1 oz., lavender 8¼ drachms. Color red with alkannin or lederin.
The usual process of manufacturing stick-pomade is as follows: Melt the fat, wax and resin in the water-bath, then strain the mixture and cool it off by constant stirring until a thin film is formed upon the surface. Then perfume and pour into tin moulds of oval, round or square form and of various sizes. A dozen of such tin moulds of the same size are generally soldered together and are provided below either with a hinged piece, or they are open. In the latter case they are placed upon a tin support with a high edge which serves for the reception for the fat escaping from any of the moulds. The cold pomades are pushed out by means of sticks of wood fitting exactly into the moulds. They are then wrapped in tinfoil, labelled and brought into commerce.
Brown wax pomade.—Best tallow 10 lbs., yellow wax 2 lbs., colored with umber. Perfume: Citronella oil 2 ozs., clove oil 12¼ drachms, bergamot oil 8¼ drachms, anise-seed oil 6¾ drachms; or, bergamot oil 2½ ozs., cassia oil 1¼ ozs., clove oil 5½ drachms.
Hair Oils.—Like pomades, hair oils are perfumed either with volatile oils or by treatment with larger quantities of fresh flowers. The oils obtained in the latter manner are known as Huiles antiques, and are the finest and most expensive. Vaseline oil, which is cheap and does not become rancid, is also at present much used as hair oil. To make the fat oils used as hair oils more durable and to protect them from becoming rancid, they are also treated with benzoin. For this purpose digest for three hours, with frequent stirring, in the water-bath 100 lbs. of the oil with 1 lb. of pulverized benzoin. With the exception of alkannin for red-colored oil and chlorophyl for herb oils, no coloring substances are used for hair oils. About 5½ to 8¼ drachms of perfume are required for 1 lb. of oil.
Peruvian bark hair oil.—Extract for some time 1 lb. of pulverized Peruvian bark with 10 lbs. of strongly heated benzoinized olive oil. Then color the oil red with alkannin, and when cold, perfume with bergamot oil 1¾ ozs., lemon oil 14 drachms, rose-geranium oil 2¼ drachms, neroli oil ½ drachm, and cinnamon oil 5 drops; or, with bergamot oil 2¼ ozs., lemon oil 1⅛ oz., geranium oil 3¼ drachms.
Fine hair oil.—Benzoated olive oil 10 lbs., lemon oil 1¼ ozs., bergamot oil 15¾ drachms, lavender oil 3¾ drachms, neroli oil 2¾ drachms, rosemary oil 1½ drachms, petit-grain oil 1 drachm.
Bandolines.—Bandolines are mucilaginous liquids, and are prepared from substances forming mucilage, such as gum-tragacanth, gum-arabic, Japanese gelatine, quince seeds, flaxseed, etc. Gum-arabic adhering very firmly, its use, however, cannot be recommended. The substances above mentioned are heated with water until the mucilaginous matter is extracted. The latter is then strained through a cloth, and the mucilaginous, thick, transparent liquid thus obtained perfumed. Volatile oils dissolving with difficulty in the liquid, an Extrait is generally used for perfuming, or an aromatic water for dissolving the gums. If the bandoline is to be colored, an ammoniacal carmine solution is to be used. Aniline colors should not be employed for the purpose, since they precipitate upon the scalp and hair, even if only traces of them are present.
Brilliantine.—Brilliantine is very popular for giving lustre to the hair of the head and the beard, and in fact, if correctly prepared, it has many advantages, since, owing to its composition, it considerably decreases, even if it does not entirely prevent, the formation of the annoying dandruff.
This brilliantine, containing no glycerin, is not so economical as the preceding. It evaporates quite rapidly and sometimes makes the hair hard, especially that of persons having naturally dry hair. However, this second quality is also quite popular and the perfumer must satisfy the demands of his customers as much as possible.
II. Almond oil 2½ lbs., spermaceti ½ lb., oil of lemon 3 ozs. Melt the spermaceti at a low temperature; add the oil and heat until all flakes disappear. Let the jars into which it is to be poured be warm, and then cool as slowly as possible to insure good crystals.
The alcohol, volatile oils, and tinctures are intimately mixed in a glass balloon, then allowed to stand two to three weeks when the distilled water is added and the whole vigorously agitated. After adding the water the fluid becomes very turbid and requires several weeks to clarify. It is then filtered through paper. If, notwithstanding filtering, it should remain somewhat turbid, bring a small quantity of carbonate of magnesia upon the filter.
The alcohol, tinctures, and volatile oils are brought into a glass balloon and after vigorous agitation allowed to stand 8 days for the volatile oils to dissolve. The decoction of Panama wood is then added, next the bicarbonate of soda solution, and finally the whole is thoroughly agitated. The Panama-wood decoction should not be added while hot, as otherwise the glass balloon might burst. Color the water with cochineal tincture or henna tincture.
Tea hair tonic.—Bay rum 2 ozs., glycerin 2 ozs., alcohol 2 ozs., infusion of black tea 10 ozs. Mix and perfume to suit. The tea infusion should be made very strong, say 1 oz. of best tea (best quality) to 10 ozs. of boiling water, let stand till cool, strain, and add the other ingredients.
Bay rum.—Genuine bay rum, as brought into commerce from St. Thomas, is said to be prepared by twice distilling a fine quality of rum with the leaves and berries of Myrcia acris or the bayberry tree. The berries are much richer in volatile oil than the leaves, but on account of the height of the trees, the gathering of the berries is connected with so many difficulties and the harvest so scanty, that the manufacturers prefer to mix leaves and berries in a certain proportion.
VI. Jamaica rum 36 ozs., 95 per cent. alcohol 36 ozs., oil of bay ½ oz., oil of pimento 1 drop, acetic ether 4 drops. Allow to stand at least 3 weeks before using.
Hair Dyes.—The requirements of a good hair-dye are that it can be readily applied, that it contains no injurious substances, and that the coloration be as natural and durable as possible. These demands are difficult to fulfil, and it cannot be said that there is one of the ordinary hair dyes which in every respect comes up to them. Black hair dyes give the most natural coloration, but the peculiar shade of blue-black hair cannot be imitated. The medium colors, light brown and blonde, are the least natural. Most dyes allow of rapid coloration, though, in order to make the deception more complete, a gradual coloration is by many persons preferred. Such gradual, though only very slightly darker coloration, is attained by the use of hair oils and certain animal fats containing a slight content of sulphur or iron, such as freshly expressed egg oil and neat's-foot oil. It was formerly believed that egg oil, if used in time, would even prevent the hair from turning gray. The gradual darkening of the hair may also be effected by agents, which are converted into colored combinations only by the atmospheric oxygen or the content of sulphur in the hair, such as extract from nut shells, tannin, pyrogallic acid and many metals, the latter chiefly in the form of pomades or hair oil. Dilute acids used for some time make the hair somewhat lighter. Mothers wishing to keep the hair of their children blonde, avoid oils, and frequently wash the heads of the children with vinegar or lemon juice. No coloration is, however, durable; it becomes in the course of time gradually weaker, and the new growth of hair always requires after-coloration.
Copper salts with certain substances, such as potassium ferrocyanide solution, potassium sulphydrate, calcium sulphydrate, and pyrogallic acid give dark-brown colorations. Of the copper salts, the sulphate in ammoniacal solution is most frequently employed, though occasionally also the chloride. These salts give a beautiful brown color to the hair. Small quantities of copper salt are also frequently added to the actual black dyes; the hair by this means acquiring a brown-black, instead of a deep black color.
Hair dyed red with henna acquires a beautiful black color when subsequently treated with indigo, this mode of dyeing black being much in vogue in the Orient. The process is as follows: The hair, being freed from fat with soap, is divided into separate strands and anointed with quite a stiff paste prepared from pulverized henna and lukewarm water. The hair, after being smoothed, is allowed to remain for at least one hour in contact with the paste, and is then rinsed off with lukewarm water. Being slightly dried, it is then in the same manner anointed with a paste prepared from indigo and water, and allowed to remain in contact with it for one hour. The hairs which were colored orange-red by the henna, now have a greenish-black appearance, but by the oxidation of the indigo in a short time acquire an intensely blue-black color, which is extraordinarily durable, the hair only after several months requiring to be again dyed.
In the following, a number of formulæ for hair-dyes are given. According to their constitution, they may be divided into two groups, viz: A. Dyes which contain the coloring matter in a finished state; and, B. Dyes which are formed upon the hair by a chemical process. The dye should first be applied in a dilute state, and the application repeated in case the desired shade is not produced, since by the use of the dye in a concentrated form a shade not resembling any natural color might be obtained, hair which is to be colored black acquiring, for instance, a metallic blue-black lustre.
Tannin hair dye. I (in the white bottle).—Pulverized gall-nuts 14 ozs., water 16 ozs., rose water 16 ozs. Boil the gall-nuts in the water, strain the boiling fluid through a close cloth into the rose water, and bring the fluid thus obtained, while still hot, into the bottles, which should be immediately closed. (It is absolutely necessary to bring the fluid hot into the bottles, as otherwise mould is readily formed.) II (in the dark bottle).—Nitrate of silver 5 ozs., distilled water 1 quart. Add water of ammonia to the silver solution until the precipitate at first formed is redissolved.
Depilatories.—While the number of agents for promoting the growth of the hair is a very small one, and their efficacy not above doubt, there are, on the other hand, quite a number of very effective agents for the removal of hair, sulphur combinations being most frequently used for the purpose. Rhusma is a depilatory which has long been known, and is still almost exclusively used in the Orient. It consists of 1 part orpiment and 6 parts of lime slaked to a powder. Mix intimately by passing the ingredients through a sieve, and preserve the mixture in tightly-closed vessels. For use, stir some of the powder to a paste with water, and apply it to the place upon which the hairs are to be destroyed. As soon as the layer of paste begins to dry remove it with a thin shaving of wood. Owing to the energetic action of this depilatory upon the skin, ladies are advised not to use it for the face.
The preparation is used for smelling-bottles. The vials are first filled with sulphate of potassa in small crystals, and enough acetic acid is added to thoroughly moisten the salt. The use of sulphate of potassa is said to have originated from the fact, that the acid mixture was formerly obtained by introducing into the vials acetate of potassa and a sufficiency of sulphuric acid. Whether this be true or not, sulphate of potassa constitutes an excellent medium for retaining the liquid in the bottle. It acts simply as an incorrodible sponge.
Rose milk (Lait de rose).—Rose water 5 lbs., white beeswax and comminuted Castile soap each 3½ ozs., potash 4½ ozs., Extrait rose No. 1 8 ozs.
The ingredients are intimately mixed and passed through a sieve. The perfumes are brought together in a glass and thoroughly shaken. The same directions hold good for all succeeding receipts for Poudre de riz. Of talc only the whitest pieces should be used, the Briancon talc or French chalk being very suitable for the purpose, it yielding a very white and delicate powder. It is prepared as follows: Over 1 part of talc pour 2 parts of vinegar, let it stand, with frequent shaking, for 14 days, then filter and thoroughly wash the talc with distilled water.
Poudre de riz muguet.—Rice flour 4 lbs., prepared talc 19 ozs., ylang-ylang oil, wintergreen oil, angelica oil, and bitter-almond oil each 2 drops, bergamot oil 5 drops, storax tincture 14 drachms, Extrait Muguet No. 1, 3½ ozs.
IV. Quince seed 1½ drachms, boric acid 4 grains, carbolic acid 10 grains, glycerin 2 ozs., alcohol 3 ozs., cologne 2 ozs., oil of lavender 20 drops, glycerite of starch 2 ozs., water sufficient to make 1 pint. Dissolve the boric acid in 8 ozs. of water, macerate the quince seed in the solution for three hours and then press through a straining cloth, add the glycerin, carbolic acid and glycerite of starch and mix thoroughly. Mix the cologne and oil of lavender with the alcohol, add the solution to the mucilage and mix the whole well.
If lip-salve of a more solid consistency is desired, the object may be attained by the addition of a few drachms of white beeswax. However, in this case, the pomade must be melted in a water-bath, or the pomade and wax melted together. Then add the carmine, stir until cold, fill into boxes and make the surface lustrous over an alcohol flame.
subnitrate of bismuth
Ordinary red paint (rouge).—Prepared talc 2 lbs., carmine 1 oz., gum-tragacanth mucilage prepared from distilled water 3½ ozs. and gum-tragacanth 2¼ drachms, best olive oil 5½ drachms, best alcohol 1 oz., spirits of sal ammoniac ½ tablespoonful, distilled water as much as required.
The procedure is now as follows: By means of a spoon bring a quantity of the paint, about the size of three hazelnuts upon the centre of a porcelain plate, spread it out uniformly to the edge of the plate by knocking the latter against the table, and in the same manner cover 6 or 8 plates. These are the test-plates. Tie a piece of paper over the dish containing the rest of the paint and set it aside. Place the plates coated with paint in a dry place to dry, but do not expose them to sunlight, nor should soaps be kept in the room, as in both cases the paint would become blue. After 12 to 18 hours the paint upon the plates will be dry, and now comes the most difficult part of the manipulation. With a small horn-knife or the sharp edge of a playing card scrape off very carefully and uniformly a small quantity from the surface of the paint, proceeding from the edges towards the centre of the plate. Then, to see whether the paint adheres firmly to the plate, knock the edge of the latter quite vigorously against the table. If it adheres firmly, cover the entire plate with a piece of watered silk, catch the ends of the latter beneath the plate with the left hand, and, with the palm of the right, run quite hard over the silk. By this means the moiré of the silk is imprinted upon the paint, giving it a nice appearance. Proceed in the same manner with the six or eight test-plates, and if the paint upon them bears the manipulation without dropping off, work up the rest of the paint in the dish. If, however, the paint does not adhere to the plates, it is proof of it containing not enough gum-tragacanth. In this case add some of the mucilage to the paint in the dish, work it thoroughly through, and proceed in the manner described. Packing, labelling, etc., being subject to fashion, need not here be described, but as the charm of novelty contributes much to the sale of an article, the manufacturer should make it his business to invent new attractive designs, without too much imitating others.
Heat the rose water, without allowing it to boil, in a glazed earthenware vessel, add the carmine, previously rubbed fine, to the hot rose water, and stir the fluid with a clean wooden spatula until the carmine is completely divided. Then take the vessel from the fire and add a tablespoonful of spirits of sal ammoniac. The latter imparts to the rouge a brighter red, but not too much of it should be used, as otherwise the rouge acquires a bluish shade, and besides the odor of the spirits of sal ammoniac is not exactly agreeable. When the rouge is cold add 1 lb. of Extrait de rose, mix the whole intimately, and filter through white filtering paper into a clean glass bottle. The rouge has to be protected from sunlight.
Besides the above-mentioned fat paints in sticks, there are also fat paints in porcelain boxes, which are of a somewhat softer consistency. They are prepared in white, rose color, and yellowish. A few receipts for them are as follows:—
A PRACTICAL TREATISE
ON THE
MANUFACTURE OF PERFUMERY.
CHAPTER I.
HISTORICAL NOTICE OF PERFUMERY.
Nature has implanted in man the instinct of finding the odor accompanying decay and putrefaction insufferable, of fleeing from it, and of going in quest of fragrant odors. Hence, in ancient times, perfume substances were highly esteemed, and an offering of them was considered a sign of the most profound reverence and homage. The early nations of the Orient especially used perfume substances in such profusion that the consumption of them by the finest lady of to-day must be called a comparatively moderate one. This may, however, be readily explained, for, on the one hand, the majority of plants which produce the most agreeable perfumes in larger quantity are indigenous to the Orient; and, on the other, the excessive exhalations from the human body, caused by the hot climate, forced the people to search for means to remove, or at least to cover, the disagreeable odor arising therefrom.
Since fragrant odors were agreeable to human beings, it was believed that they must be welcome also to the gods, and, to honor them, perfume substances were burned upon the altars. Besides, as an offering to the gods, perfume substances were extensively used by many nations, especially by the Egyptians, for embalming the dead, the process employed by the latter having been transmitted to us by the ancient authors Herodotus and Diodorus.
Furthermore, a desire for ornamentation and to give to the face and body as pleasing an appearance as possible, is common to all mankind. To be sure, the ideas of what constitutes beauty in this respect have varied at different times and among the various nations. But, independent of the savage races, who consider painting and tattooing the body and face an embellishment, and taking into consideration only the earliest civilized nations, it is astonishing how many arts of the toilet have been preserved from the most ancient historical times up to the present. "In the most ancient historical times, people perfumed and painted, frizzed, curled, and dyed the hair as at present, and, in fact, the same cosmetics, only slightly augmented, which were in use hundreds, nay, thousands, of years ago are still employed to-day."[1] It is especially woman, who everywhere exercises the arts of the toilet, while, with the exception of perfumes and agents for the hair, man is but seldom referred to as making use of cosmetics. The young girls of ancient Egypt used red and white paints, colored their pale lips, and anointed their hair with sweet-scented oils; they dyed their eyelashes and eyelids black to impart a brighter lustre to the glance of the eye, and the mother of the wife of the first king of Egypt is said to have already composed a receipt for a hair-dye.
From the Egyptians, the practices of the toilet, like many other things, were transmitted to the Jews. In Egypt, the Hebrew woman had known the sweet-scented flower of the henna bush, and, finding it also in Judea, it served her as a perfume. In the Bible the henna flower is called kopher, in Greek kypros, and the Cyprian salve, mentioned by Pliny, was prepared by boiling henna flowers in oil and then expressing them.
Painting the face was also practised by the Hebrew women, reference being made to it in II. Kings ix. 30, and Jeremiah v. 30, while painting of the eyes is mentioned in Ezekiel xxiii. 40.
The number of perfume substances known to the ancient Hebrews was but a limited one, they consisting, besides the above-mentioned henna flower, chiefly of a few gum-resins, especially bdellium, olibanum and myrrh.
In ancient times olibanum was, without doubt, the most important perfume-substance. It was introduced into commerce by the Phoenicians, and, like many other substances, it received from them its name, which was adopted by other nations. Thus, the Hebrews called the tree lebonah, the Arabs, lubah, while the Greeks named it, λιβανός and the resin derived from it, the celebrated frankincense of the ancients, λιβανωτόςτς, Latin, olibanum. Regarding the mode of gaining the olibanum, some curious ideas prevailed in ancient times. Thus, Herodotus writes: "Arabia is the only country in which olibanum grows, as well as myrrh, cassia, cinnamon and lederum. With the exception of myrrh, the Arabs encounter many difficulties in procuring these products. Olibanum they obtain by burning styrax, for every olibanum tree is guarded by a number of small-sized winged serpents of a variegated appearance, which can be driven away by nothing but styrax vapors." According to Pliny, who gives a very full account of olibanum, Arabia felix received its by-name from the abundance of olibanum and myrrh found there. He states that olibanum grows in no other country besides Arabia, but it is not found in every part of it. About in the centre, upon a high mountain, he continues, is the country of the Atramites, a province of the Sabeans, from which the olibanum region is distant about eight days' journey. It is called Saba and is everywhere rendered inaccessible by mountains, a narrow defile, through which the export is carried on, leading into an adjoining province inhabited by the Mineans. In Saba itself were not more than 300 families, called the saints, who claimed the cultivation of olibanum as a right of heritage. When making the incisions in the trees, and while gathering the olibanum, the men were prohibited from having intercourse with women and from attending funerals. Notwithstanding the fact that the Romans carried on war in Arabia, none of them had ever seen an olibanum tree. When there was less chance of selling the olibanum, it was gathered but once in the year, but since the increase in the demand, it was gathered twice, first in the fall and again in the spring, the incisions in the trees having been made during the winter. The collected olibanum was brought upon camels to Sabota, where one gate was open for its reception; to turn from the road was prohibited under penalty of death. The priests took one-tenth by measure for the god Sabin, sales not being allowed until their claim was satisfied. The olibanum could be exported only through the territory of the Gebanites, whose King also levied tribute.
Pliny further states that the Arabs did not steal one from another, but for fear of loss those employed in the stores of Alexandria were forced to go naked with the exception of a clout which was sealed. A mask and a thick net were thrown over the head.
To us the practice of anointing the entire body, customary among the ancients, appears very singular. Old Egyptian sculptures represent the guests being anointed at the meal. Among the Jews we find a holy oil with which Aaron and his sons were anointed to consecrate them to the priesthood, Moses prescribing for this holy anointing oil, myrrh, cinnamon, calamus, and oil from the olive tree. Other persons were prohibited from imitating or using this holy oil. The anointing of kings was introduced later on. Though it was prohibited to imitate and use the holy oil, this prohibition did not refer to anointing with oil in general.
That the Greeks also set a high value upon anointing with oil is plainly seen from Homer. When Telemachus visited Nestor, Polycaste, Nestor's youngest daughter, bathed him and anointed him with oil, and when he was the guest of Menelaus, the maids of the latter performed the same service for him, while for Ulysses returning as a beggar, the aged Euryclea prepared a foot-bath and anointed him.
By the addition of fragrant substances to the oil, the sweet-scented ointment, myron, originated. While the anointing with simple oil evidently served as a hygienic measure after the bath, and especially for men in the gymnasium, and before a combat, with the Greeks, ointments were an article of luxury. In Socrates' time the use of sweet-scented ointments had reached such an extent, that Xenophon caused him to speak against it, but, as is the case with all such lectures against fashion, without the slightest success. In Athens the luxury was carried so far that the bacchanalians anointed each part of their body with a special ointment. The oil extracted from the palm was thought best adapted to the cheeks and the breasts; the arms were refreshed with balsam-mint; sweet marjoram supplied an oil for the hair and eyebrows; and wild thyme for the knee and neck. Although to us it would be repugnant to have the entire body anointed, in Athens it was considered beautiful to be glossy with ointments. It is said of Demetrius Phalereus, that in order to appear more captivating, he dyed his hair yellow, and anointed the face and the rest of his body.
From the Asiatics and Greeks the Romans also learned the use of ointments. Pliny cannot say at what time they were introduced in Rome, but states that after the conquest of Asia and the defeat of the King, Antiochus, in the year 565, after the building of Rome, the censors issued an edict prohibiting the sale of foreign ointments. However, this edict was of no use, and the practice spread more and more, Pliny speaking very bitterly about it. Regarding this extravagance in ointments, Plutarch says: "Frankincense, cinnamon, spikenard, and Arabian calamus are mixed together with the most careful art and sold for large sums. It is an effeminate pleasure and has spoiled not only the women but also the men, who will not sleep even with their own wives if they do not smell of ointments and powders." Plutarch further mentions an incident which must have created a sensation even in luxurious Rome, as otherwise it would scarcely have been chronicled for the benefit of posterity. Nero one day anointed himself with costly ointments and scattered some of them over Otho. The next day Otho gave Nero a banquet, and laid in all directions gold and silver tubes, which poured forth expensive ointments like water, thoroughly saturating the guests.
Directions for preparing ointments are contained in Theophrastus's work "On Perfumes," in Dioscorides's "Medica materia," and Pliny's "Historia naturalis." Dioscorides's receipts are the fullest. According to Pliny, a distinction was made between the juice and the body, the latter consisting of the fat oils and the former of the sweet-scented substances. In preparing the ointments, the oil together with the perfuming substances were heated in the water-bath. For instance, rose ointment was, according to Dioscorides, prepared by mixing 5½ lbs. of bruised Andropogon Schœnanthus with a little water, then adding 20½ lbs. of oil and heating. After heating the oil was filtered off, and the petals of one thousand roses were thrown into the oil, the hands with which the rose leaves were pressed into the oil being previously coated with honey. When the whole had stood for one night, the oil was strained off and when all impurities had settled, it was brought into another vessel and fresh rose leaves introduced, the operation being several times repeated. However, according to the opinion of the ancient ointment makers, no more odor was absorbed by the oil after the seventh introduction of rose leaves. To fix the odor, resins or gums were added to the ointments.
A process of distilling volatile oils was also known, the odoriferous matter being caught by spreading wool over the heated perfume-substances. The wool was afterwards subjected to pressure. This process, of course, involved great loss and was available only for substances containing much volatile oil.
Dioscorides also gives directions for making animal fats suitable for the reception of perfumes. Beef-tallow, deer-fat, or the marrow of animals was freed from all membranes, melted together with a little salt in an entirely new vessel, and then poured into clean water, where it was washed by rubbing with the hands, the water being frequently renewed. Then it was boiled with equal parts of sweet-scented wine, and after taking it from the fire it was allowed to stand over night. The next day the cold fat was again boiled in a new vessel, with sweet-scented wine, this operation being repeated until the fat had lost every trace of disagreeable odor, when it was brought in contact with the perfumes.
The consumption of perfume-substances by the ancient Romans must have been enormous. The trade of the ointment makers (ungentarii) was so extensive that the large street Seplasia in old Capua was entirely taken up by it, and the business must have paid well since the prices realized were very high. However, in ancient times the business cannot have been very agreeable, at least not in Greece, as shown by a passage in Plutarch's Life of Pericles: "We take pleasure in ointments and purple, but consider the dyers and ointment makers bondsmen and mechanics."
Red and white paints, in the form of powder as well as of paste, were extensively used by the Roman ladies. Chalk and white lead served for white paint, and minium and carmine for red. Lovers preferred white paints, a pale color being more becoming to them:—
"Palleat omnis amans; hic est
color aptus amanti."—(Ovid.)
For black paints for the eyebrows roasted ant eggs or soot were used.
The Roman ladies paid as much attention to their natural, and also false, hair as the fair ones of to-day. They curled their hair with heated iron instruments, and perfumed them with fragrant oil. If from age, sorrow, or other reasons, the hair was no longer black, it was dyed, and it seems that a considerable number of hair-dyes were known in Rome, amongst them some which are still employed to-day, such as green nutshells and acetate of lead.
After the Romans had seen the blonde German maidens, blonde and red hair became the fashion. To dye the hair blonde sharp alkaline soaps were chiefly used. However, this or some other hair-dye seems to have been very injurious, as it caused the hair to come out. The satirists ridiculed this as well as the wigs, which were worn by men and women to hide baldness, or on account of the color which could not be attained by dyes.
Depilatories were also known to the Romans, the agents employed being called psilothrum and dropax. They were of vegetable origin, but it is not exactly known from which plants they were derived.
For cleaning the teeth the Roman ladies used a dentifrice which does not seem very inviting to us. It consisted of a urine imported from Spain (dens hiberna defricatus urina). To perfume the breath or to hide its bad odor, mouth-washes, perfumed with saffron, roses, etc., were used, or myrrh, mastic from Chios or perfumed pastilles were chewed.
We know but little regarding the use of perfumeries and cosmetics in the Middle Ages. In the wars during the migrations of the nations, but little thought was very likely given to them, but as soon as the nations became again settled and made sufficient progress in culture, the taste for perfumes and other pleasures of life no doubt returned. Our knowledge in this respect is limited to what is contained in the works of physicians of the first centuries. Later on we find receipts for cosmetics in the writings of Arabian physicians, such as Rhazes (end of the 9th to the commencement of the 10th century), Avicenna (end of the 10th to the commencement of the 11th century), and Mesuë (11th century). To the 11th century also belong the works of the celebrated Trotula, "De mulierum passionibus," "Practica Trotulae mulieris Salernitanae de curis mulierum," and "Trotula in utilitatem mulierum," all of which contain receipts for cosmetics. In the 14th century the most celebrated surgeon of the Middle Ages, Guy de Chanlios, did not consider it beneath his dignity to devote a section of his "Grande Chirurgie" to cosmetics. However, it was only in the 16th century that perfumes and cosmetics came again into prominent notice in Italy, which at that time was the country of luxury and art. Giovanni Marinello,[2] a physician, in 1562 wrote a work on "Cosmetics for Ladies," which he dedicated to the ladies Victoria and Isabella Palavicini. In the preface the author expresses the opinion that it is only right and pleasing to God to place the gifts bestowed by him in a proper light and to heighten them. He then proceeds to give perfumes for various purposes, aromatic baths to keep the skin young and fresh, means for increasing the stoutness of the entire body and of separate limbs, and others for reducing them. He further recommends certain remedies for making large eyes small, and small ones large. The chapter on the hair is very fully treated. To prevent the hair from coming out, rubbing with oil, and then washing with sorrel and myrobalan is recommended. For promoting the growth of the hair, the use of dried frogs, lizards, etc., rubbed to a powder, is prescribed. Means for making the hair long and soft and curly are also given, and others recommended for eyebrows and eyelashes. As depilatories lime and orpiment are prescribed. Paints are also classed among general cosmetics. Their use became at this time more and more fashionable, and not only the face, but also the breast and neck were painted.
Catherine of Medici and Margaret of Valois introduced these arts of the toilet into France. That country soon became the leader in this respect, and for many years the greatest luxury in perfumes and cosmetics prevailed there. The golden age for these articles lasted from the commencement of the seventeenth to the middle of the eighteenth century, during which time the mouche or beauty patch also flourished. "There were at that time hundreds of pastes, essences, cosmetics, a white balsam, a water to make the face red, another to make a coarse complexion delicate, one to preserve the fine complexion of lean persons and again one to make the face like that of a twenty-year old girl, an Eau pour nourir et laver les teints corrodés and Eau de chair admirable pour teints jaunes et bilieux, etc. Then there were Mouchoirs de Venus, further bands impregnated with wax to cleanse and smooth the forehead; gold leaf was even heated in a lemon over a fire in order to obtain a means which should impart to the face a supernatural brightness. For the hair, teeth and nails there were innumerable receipts, ointments, etc. However, of special importance were the paints, chemical white, blue for the veins, but, chief of all, the red or rouge, mineral, vegetable, or cochineal. The application of rouge was at that time no small affair, it was not only to be rouged, but the rouge had also to express something—Le grand point est d'avoir un rouge qui dise quelque chose. The rouge had to characterize its wearer; a lady of rank did not wear the rouge like a lady of the court, and the rouge of the wife of the bourgeois was not like either of them nor like that of the courtesan. At court a more intense rouge was worn, the intensity of which was still increased on the day of presentation, it being then Rouge d'Espagne and Rouge de Portugal en tasse. It may seem incredible, but for eight days a violet paint was used and then for a change Rouge de Serkis. Ladies, when retiring for the night applied a light rouge (un demi rouge), and even small girls wore rouge, such being the decree of fashion. The ladies dyed their eyebrows and eyelashes, and powdered their hair, both natural and false, for, about 1750, they commenced wearing wigs and chignons. Powdering was done partially for the purpose of dying the hair after dressing, and partially for decoration; white, gray, red and fiery red powders were in vogue."
To that time fashion also ordained an ever-varying routine in the employment of perfumes; so that the royal apartments were one day fragrant with the scent of the tuberose and the next with that of amber and cloves; and so on consecutively, each succeeding day bringing a change of the reigning odor. In that luxurious age the personal use of perfumes was not confined to the fair sex, but the effeminate gallants of the day gloried in perfuming themselves with the favorite scents of their mistresses or of prominent belles; so that the allegiance was recognized, not as in more chivalrous times by the knight wearing the colors of the fair one who had enslaved him, but by his smelling of the particular odor which she had consecrated to herself.
Philip Augustus, in 1190, granted a charter to the French perfumers, who had formed a guild. This charter was, in 1357, confirmed by John, and in 1582 by Henry III., and remained in force until 1636. The importance of the craft in France is shown by the fact that under Colbert the perfumers or "parfumeurs-gantiers," as they were called, were granted patents which were registered in Parliament. In the seventeenth century Montpellier was the chief seat of the French perfumery industry; to-day it is Paris, and over fifty millions of francs' worth of perfumery are annually sold there. The parfumeurs-gantiers had the privilege of selling gloves of all possible kinds of material, as well as the leather required for them; they had the further privilege of perfuming gloves and selling all kinds of perfumes. Perfumed leather for gloves, purses, etc., was at that time imported from Spain. This leather was very expensive and fashionable, but on account of its penetrating odor its use for gloves was finally abandoned.
In England perfumes were not in general use before the reign of Queen Elizabeth, when they soon became fashionable. Elizabeth had an especially finely developed sense of smell and nothing was more repugnant to her than a disagreeable odor. She had a cloak of perfumed Spanish leather, and even her shoes were perfumed. Perfumed gloves were also fashionable. The city soon imitated the practices of the court, and that an extravagant use was made of perfumeries and cosmetics is plainly seen from the works of the authors of that time, as well as from an act of Parliament passed in 1770. By the latter it is ordained that any woman, no matter of what age or rank, be she maid or widow, who deceives a man and inveigles him into matrimony by the use of perfumeries, false hair, Crépons d'Espagne (a paint), corsets, hooped petticoats, shoes with high heels, and false hips, shall suffer the penalty of the law for procuring, and the marriage shall be null and void.
[1] Paschkis, Kosmetik für Aerzte. Wien, 1890.
[2] Gli ornamenti delle donne, tratti dalle seriture d'una Reina greca, par M. Giovanni Marinello in Venetia.
CHAPTER II.
THE PERFUME-MATERIALS FOR THE MANUFACTURE OF PERFUMERY.
Most of the perfume-materials employed by the perfumer are derived from the vegetable kingdom; a few are of animal origin, whilst some are artificially prepared.
Of animal substances only four are used, namely: musk, castor or castoreum, civet, and ambergris; the separation of their characteristic odoriferous substances has, however, not yet been accomplished. The odor of plants is generally due to volatile substances called volatile or essential oils. Their occurrence is not limited to special parts, they being found in the flower, seed, wood, bast, bark, leaves, and root. However, in every plant the oil occurs chiefly in certain organs, and it even happens that the oil differs with the part of the plant whence it is derived. The odors exist already formed in the living plant, or else are generated, as in the instance of bitter almonds, by some reaction between the elements which takes place during fermentation or distillation.
From the strength of the odor of a plant no conclusion can be drawn as to the quantity of volatile oil present. If this were the case, the hyacinth, for instance, would contain more oil than the coniferae, whilst in fact it contains so little that it can be separated only with the greatest difficulty. The odor does not depend on the quantity, but on the quality of the oil; a plant may diffuse but little odor and still contain much volatile oil. Of the various families of plants, the labiatae, umbelliferae, and coniferae are richest in volatile oils.
In every climate plants diffuse odor, those growing in tropical latitudes being more prolific in this respect than the plants of colder regions, which, however, yield the most delicate perfume. Although the East Indies, Ceylon, Peru, and Mexico afford some of the choicest perfumes, Central Europe is the actual flower garden of the perfumer, Grasse, Cannes, and Nice being the principal places for the production of perfume-materials. Thanks to the geographical position of these places, the cultivator, within a comparatively narrow space, has at his disposal various climates suitable for the most perfect development of the plants. The Acacia Farnesiana grows on the seashore, without having to fear frost, which in one night might destroy the entire crop, while at the foot of the Alps, on Mount Esteral, the violet diffuses a much sweeter odor than in the hotter regions, where the olive and the tuberose reach perfect bloom. England asserts its superiority in oils of lavender and peppermint. The volatile oils obtained from plants cultivated in Mitcham and Hitchin command a considerably higher price than those from other localities, this preference being justified only by the delicacy of their perfume. Cannes is best suited for roses, acacias, jasmine, and neroli, while in Nimes, thyme, rosemary, and lavender are chiefly cultivated. Nice is celebrated for its violets, while Sicily furnishes the lemon and orange, and Italy the iris and bergamotte.
The odors exhaled by our own domestic plants have been but little studied, but the southern as well as many northern districts of the United States are well adapted for the cultivation of quite a number of species of plants which might be made to yield highly valuable articles of commerce. Among the plants which might furnish oils for the perfumer's use are, for instance, the wall flower, the Lilly, lilac and mignonette.
Volatile Oils.—The volatile oils are either fluid (actual volatile oils) or solid (varieties of camphor) or solutions of solid combinations in fluid. The latter, on exposure to low temperatures, separate into two portions, one solid, called stearoptene, and the other liquid, called elæoptene. The boiling point of the volatile oils is considerably higher than that of water, but when heated with water they pass over with the vapors. Upon paper, fluid volatile oils produce grease spots, which differ, however, from those caused by fat oils in that they gradually disappear at an ordinary temperature, and rapidly by gentle heating. Most volatile oils are insoluble, or only with difficulty and sparingly soluble, in water, but they impart to the latter their odor and taste. They are readily soluble in alcohol, ether, chloroform, bisulphide of carbon and petroleum-ether, and miscible in every proportion with fats and fat oils. By their solubility in alcohol they differ from most fat oils. When freshly prepared many volatile oils are colorless, but soon turn yellow; some, however, show a distinct color even when fresh. They ignite with greater ease than fat oils and burn with a fierce smoky flame depositing a large amount of carbon. They exhibit a great tendency to absorb oxygen from the air and to gum, the influence of light promoting the process. In specific gravity they range from about 0.75 to 1.17, most of them being specifically lighter than water. Most bodies, under otherwise equal conditions, show always exactly the same specific gravity, the variations being so slight that they may be justly ascribed to errors of observation. However, one and the same volatile oil frequently shows such variations in specific gravity, that we are forced to ascribe this phenomenon to alterations in the constitution of the oil itself. For the exact determination of the specific gravity of a volatile oil, it should, therefore, be subjected to examination immediately after its preparation from the plant or vegetable substance, which should be as fresh as possible. The influence of light upon volatile oils is best shown by the following interesting experiment: If certain volatile oils are distilled in a vacuum or over burnt lime in a current of carbonic acid, it is no longer possible to distinguish, for instance, oil of lemon from oil of turpentine; however, by again exposing the oils to the air, they reacquire their characteristic odor.
According to their elementary composition the volatile oils may be divided into three principal divisions:—
1. Volatile oils free from oxygen, terpene (camphene), or hydrocarbons.
2. Oxygenated volatile oils.
3. Volatile oils containing sulphur.
On account of the facility with which most of the volatile oils absorb oxygen, oils originally free from oxygen are frequently a mixture of hydrocarbons and combinations containing oxygen. The volatile oils varying so much in their physical as well as their chemical properties, a suitable classification of them has thus far been unsuccessful.
Most of the volatile oils contain a liquid hydrocarbon, terpene, which is characterized neither by special taste nor odor, nor is the peculiarity of a volatile oil dependent on it. In the direct distillation of a volatile oil, for instance, lemon oil, this hydrocarbon (citrene), passes first over and can, therefore, be readily separated from the constituents on which depend the peculiarity of lemon oil, and which distil over at a higher temperature. The specific character of an oil is generally due to the portion of the oil containing oxygen. Hence, manufacturers have endeavored to free several of the volatile oils, used for perfumery and the preparation of food, from the worthless terpene and at the same time to obtain them in a concentrated form. Carvol is, for instance, caraway oil freed from carvene (terpene). These concentrated oils are not only purer and more agreeable in odor and taste and more readily soluble in dilute alcohol, but, being more concentrated, an equal volume of them goes much further than ordinary volatile oil. In the price lists these oils are designated as extra strong, patented, concentrated, highly concentrated oils or essences.
All the terpenes occurring in the various oils are combinations having the formula C10H16, or polymeric with it, C15H24, C20H32, etc. These terpenes exhibiting certain deviations in regard to their properties, odor, specific gravity, and boiling points, nearly as many terpenes as there are volatile oils have been distinguished. It is, however, very likely that these deviations may be traced back to fortuitous circumstances, for example, to the admixture of foreign substances occurring together with the terpenes, and that, by a more accurate examination, the number of terpenes entitled to be considered pure chemical combinations will be considerably reduced. By Wallach's labors, the identity of several terpenes formerly considered distinct, has already been established, whilst many others have been found to possess properties in common.
According to the nature and quantity of the odoriferous substances contained in the plants, various methods, namely, expression, distillation, extraction, maceration, and absorption, are employed for the purpose of obtaining them.
Expression.—This is only practicable when the substances are especially rich in oil and of sufficient softness, as in the case with the peel of the orange, citron, lemon, etc. In such instances the material is simply placed in a linen cloth and subjected to a strong pressure until it ceases to yield oil. The press may be of any size according to the quantity to be expressed. For small quantities it generally consists of an iron vessel, having a small opening at the bottom so that the oil may flow out. The material is placed upon a perforated bottom inside of the vessel and covered with a well-fitting iron plate, that can be pressed down by means of a screw. Though the material is fairly exhausted by such a press, for large operations it is advisable to make use of a hydraulic press, which is constructed and managed in exactly the same manner as those used for the expression of fixed oils.
By expression a turbid milky fluid is obtained, which consists of the volatile oil and aqueous substances. The latter are a solution of various extractive substances and salts in water. This fluid, as it runs from the press, is received in tall, narrow, glass vessels and brought into a cool place for clarification. This frequently requires several days, three distinct layers being generally distinguished. On the bottom is a mucous layer consisting of cell substances carried along by the liquid bodies. Over this is a clear fluid consisting of a solution of extractive substances, vegetable albumen, and salts, and upon this floats the volatile oil, being specifically the lightest body, which, by its greater refractive power, can be clearly distinguished from the aqueous fluid.
The oil is separated by bringing all that has been expressed into a bottle provided near the bottom with a lateral neck closed by a cock. After separating the oil from the aqueous fluid, the latter is allowed to escape by opening the cock.
The oil obtained in this manner is still impure, and requires further treatment to remove small vegetable fibres, invisible to the naked eye, which float in them, and cause them to be somewhat opaque and slightly opalescent. By their subsequent decomposition they would also give the oil a disagreeable odor.
There are two methods of obtaining the oil entirely clear, viz., filtration and distillation. Filtration is the cheaper process, but requires special precautions to exclude the air as much as possible to prevent the oil from undergoing injurious changes. By arranging the filtering apparatus so that the oil always comes in contact with only the same quantity of air, the injurious action of the oxygen is reduced to a minimum. It is self-evident that the apparatus should not be placed in the sun, but in a semi-dark, cool place.
Fig. 1.
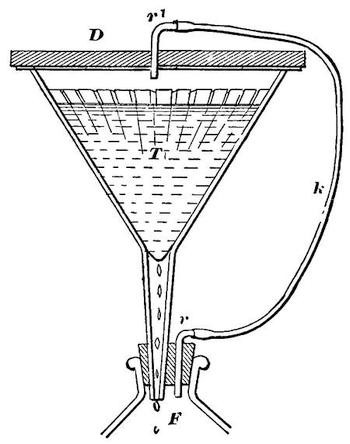
A filter of simple construction, and performing excellent service, is shown in Fig. 1. It consists of a large glass bottle, F, hermetically closed by a doubly-perforated cork. The neck of the glass funnel T, the upper rim of which is ground smooth, is placed in one of the holes, and a glass tube, r, bent at a right angle, is fitted into the second hole. A thick wooden lid, with a rubber ring on the lower side, is placed upon the funnel, thus closing it air-tight. In the centre of the lid is fitted a glass tube, r´, also bent at a right angle, which is connected with the tube r, by a rubber hose, k. After the funnel has been provided with filtering paper and the oil to be filtered, the lid is placed upon it, and must not be removed, except for the purpose of pouring more oil into the funnel. The air in the bottle F is displaced by the oil dropping into it, and escapes through r, k and r´ into the funnel, and thus only the air in the bottle and funnel can act upon the oil.
The other method for the complete purification of expressed oil is by rectification or distillation with water. For this purpose the oil, together with a little water, is brought into one of the stills described later on, and the oil distilled over. It is sometimes difficult to obtain the last portion of the oil, especially with a still heated by direct fire, and it is therefore preferable to combine it with a fresh quantity of the same oil to be distilled.
Distillation.—There are at present two methods in use. The one is founded upon the direct action of the heat, the other upon the use of steam. The first was formerly in general practice, and is still largely employed in France and England, and to a limited extent in this country. It is, however, very deficient in many respects. As the stills must necessarily be of small capacity, only small quantities can be distilled at one time, and the oils very rarely possess the peculiar odor due to them, and sometimes the odor is even altered. In mixing too little water with the materials to be extracted, there is danger that empyreumatic oils will be formed; a large quantity of water, on the other hand, is of disadvantage, in so far as in case the perfume-materials contain little oil, only a perfumed water, but no oil, will be obtained. In order to avoid these inconveniences, or, at least, to do away with some of them, another plan was devised. The materials to be distilled were spread upon sieves, which were suspended in the upper part of a still, so that they might be penetrated from below. It is true no scorching is possible in this case, as was in the other process when the heating was continued after all the water had evaporated, and the oil retains its proper color, but by this method only small quantities can be extracted at a time. The still generally used for distillation with direct heat resembles so much an ordinary whiskey still as to need no further description here.
Fig. 2.
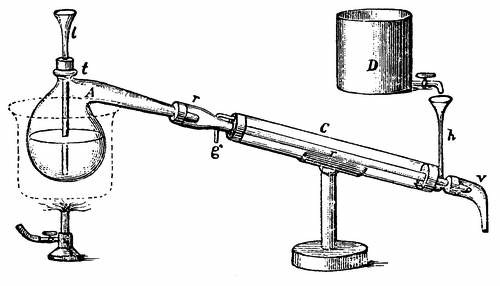
For the accurate determination of the percentage of volatile oil a vegetable substance will yield, or to obtain the oil from very costly raw materials, the small glass apparatus, Fig. 2, is used. The flask A, with a capacity of up to 5 or 6 quarts, serves for a still. In the tube t, shaped like the neck of a bottle, is inserted by means of a cork, a funnel tube, l, reaching to the bottom of the flask. The neck of the flask passes into the cooling pipe, which lies in a so-called Liebig cooler. This consists of a wide-glass tube, C, into the lower end of which, at h, flows cold water from the reservoir D, displacing the heated water at g. The lower end of the cooling pipe is connected with the neck-shaped tube v, under which stands the vessel for the reception of the distillate. To prevent the cracking of the flask, which might readily happen with the use of direct heat, it is placed in a vessel filled with sand or water.
Fig. 3.
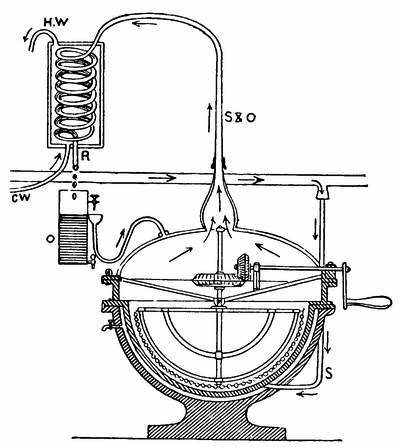
A very good small apparatus for the distillation of volatile oil is shown in Fig. 3. It is known as a siphon still. It consists of a double-walled boiler, surmounted by a still-head, which is provided with a mechanism for keeping the contents of the boiler in motion. This stirring apparatus consists of a perpendicular shaft, bearing a frame work of iron, curved so as to correspond to the interior shape of the still, and on the outside carrying a chain which scrapes over the inner surface of the still while the stirrer is being turned. This may be done either by hand or by steam. The still having been charged with the material to be extracted, is filled up with water to within a few inches of the top of the body of the still, and the latter is heated by admitting steam. The vapors arising are conducted to a cooler situated at a higher level than the still itself, and the condensed liquid is collected in a receiver, where the oil and water separate. This receiver is provided with two faucets, one near the top and the other near the bottom. If the oil passing over is heavier than water, the excess of the latter is removed by the upper faucet; if the oil swims on the water, the lower faucet is regulated so as to allow the water to escape in about the same ratio as it enters the receiver. In either case the condensed water is made to run back into the still, and the loss of oil is, therefore, greatly reduced.
Sometimes a single-walled still is used, and distillation carried on with direct steam. This method is, however, not suitable where the presence of water is necessary, for instance, in the production of oil of bitter almonds.
A simple way of converting an ordinary still into use with steam is shown in Fig. 4. For the helmet of the still A is substituted a cylindrical vessel, B, with an opening in the bottom. The materials to be distilled are brought into B, and rest upon a wire bottom to prevent particles from falling into A. From the upper portion of B a pipe, R, leads to the condenser. As may be seen from the illustration, the still A serves only for the generation of steam. The latter, in passing through B, heats the contents and absorbs the liberated oil, the combined vapors passing into the condenser.
Fig. 4.
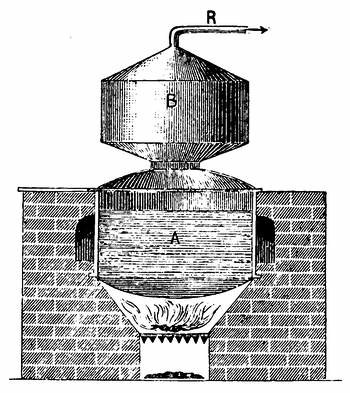
This simple modification of the ordinary still affords some advantage, the principal being the avoidance of the condensation of a large quantity of water. This in itself would not amount to much, but it has to be taken into consideration that, though volatile oils are only very sparingly soluble in water, they are nevertheless soluble in it to such a degree as to impart to it their characteristic odor and taste. Such aromatized water can be utilized in the manufacture of liqueurs and perfumery, but to the manufacturer who restricts himself to the production of volatile oils alone, this represents a loss, and it is therefore necessary for him to condense as little water as possible. And this object can only be attained by the use of direct steam.
A simple apparatus for the purpose is shown in Fig. 5. The still b, provided with a helmet, rests free upon a suitable support. To prevent cooling, it is surrounded with a wooden jacket, M. The material to be extracted rests upon a perforated bottom, beneath which enters the pipe HD, which conducts the steam from the boiler. For the more uniform distribution of the steam, it is recommended to let this pipe end in a perforated coil. The water condensed in the apparatus itself is discharged through the short pipe H, placed in the lowest part of the still.
Fig. 5.
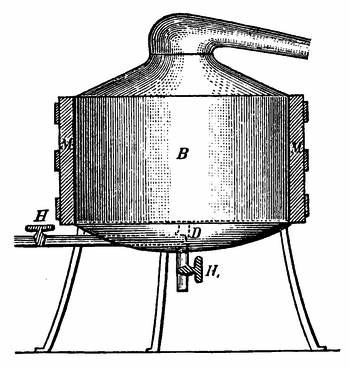
An improved apparatus for distilling dry substances by steam has been patented in Germany by Messrs. Schimmel & Co., of Leipzic. The tall conical column at the left (Fig. 6) is the still. About eight inches from the bottom is a perforated diaphragm or false bottom, upon which the material to be distilled is placed by introducing it through the still-head. A perforated coil below the diaphragm projects steam upwards through the mass, which is occasionally agitated from without by means of a horizontal stirring apparatus indicated by the two crosses. Any condensed water which may run back is converted into steam by the heating coil at the bottom. Meanwhile, the mass itself is heated by a long coil lining the body of the still and carrying steam at a high pressure. Whatever of volatile oil is carried forward by the steam passes through the still-head into the cooler on the right, where both oil and steam are condensed, and from where they flow through a small funnel tube into three successive receivers, which are arranged like Florentine flasks, and which retain the volatile oil that has separated. From the last receiver the water, which is still impregnated with oil, enters another reservoir, shown in the illustration only by dots, and from there it flows into a small globular still situated underneath; in which, by means of steam, nearly all the oil still retained is again volatilized with the steam of the water and both again conducted to the cooler.
Fig. 6.
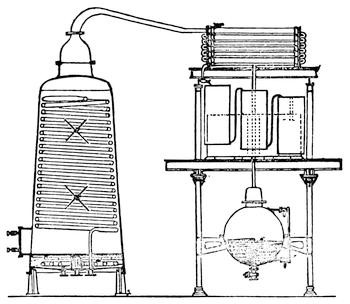
Attempts have been made to effect the distillation of volatile oils without the use of steam by means of hot air, but comparative experiments have shown that less oil is obtained. With the use of steam, the vegetable substances swell up by the absorption of water, and thus afford a free passage to the oil, liberated from the sacs containing it. With the use of hot air, on the other hand, the surface of the plant is completely dried and shrivels to a hard solid mass, which offers considerable resistance to the process of distillation.
This injurious effect of hot air can be somewhat overcome by thoroughly moistening the plants to be distilled, and allowing the hot air, before entering the still, to pass through a pipe filled with sponges constantly kept wet. But this process offers no advantages over that by steam. The apparatus required is far more complicated; and, besides, a ventilator has to be provided for forcing the hot air through the apparatus.
Separation of the oil and water.—As previously mentioned the specific gravity of most volatile oils is less than that of water. This behavior is utilized for the separation of the oil and water, by means of a so-called Florentine flask (Fig. 7). It consists of a glass flask provided near the bottom with a pipe, a, rising vertically to near the neck c of the flask where it is bent downwards as shown in the illustration. The mixed liquid of water and oil drips from the cooling pipe into the flask, and the water w, being specifically heavier, separates from the oil floating on the top, and gradually ascends in the pipe a, finally flowing over at d. Oils specifically heavier than water are caught in receivers provided with a discharge-pipe near the mouth of the flask as shown in Fig. 8.
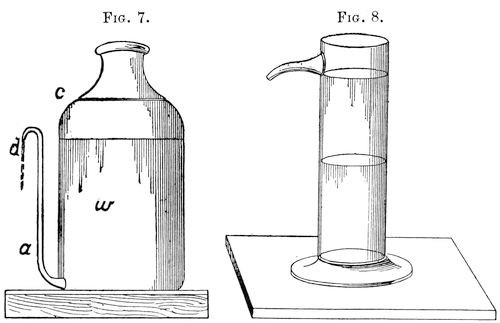
The oil delivered from the receivers is, however, still mixed with some water, dirt, etc., and for their separation is allowed to stand quietly for some time. The final separation is effected either by simply pouring off the oil, especially if larger quantities have to be handled, or with the assistance of a separator-funnel (Fig. 9). This consists of the glass-funnel T secured to the stand G, and provided with a close-fitting lid, P. The fluid is poured into the funnel, the lid placed in position, and the whole allowed to rest until the water W is completely separated from the oil O. The oil is then separated from the last drops of water by carefully opening the faucet H.
Most volatile oils are obtained by distillation, but this method is not practicable for separating the odoriferous principle of many of the most sweet-scented and delicate flowers, partially because the flowers contain too little oil, and partially because the oil would lose in quality if obtained by distillation.
Fig. 9.
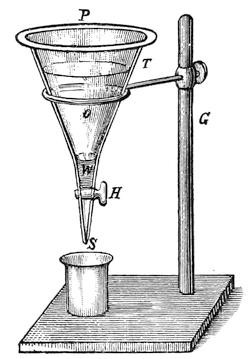
Extraction.—For obtaining the volatile oils by extraction various solvents such as ether, bisulphide of carbon, etc., may be employed. Carefully rectified petroleum-ether is very suitable for the purpose. It completely evaporates at about 122° F., and when sufficiently purified does not possess a disagreeable odor. The process of extraction is briefly as follows: The material to be extracted is treated in a digester with petroleum-ether or one of the above-named solvents. The solution is then drawn off and the solvent evaporated in a still. The recondensed solvent flows immediately back into the digester and further extracts the material contained therein. The operation is repeated until nothing soluble remains. In practice some difficulties are, however, connected with this process since, besides the volatile oils, resins, and coloring and extractive substances are dissolved, which have to be removed, as well as the last traces of the solvent, as otherwise the oil would acquire a foreign odor. Further the solvents mentioned are very volatile and inflammable, requiring the greatest precautions as regards fire. For these reasons the extraction process is not suitable for many purposes, and though at first great hopes were entertained in regard to it, its use is limited to substances with a large content of volatile oil.
Fig. 10.
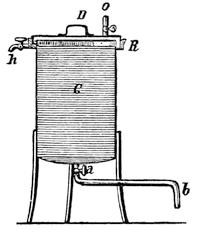
For extraction on a small scale, the apparatus, Fig. 10, is a very suitable one. It is especially adapted for manufacturers of perfumery, who wish to extract fresh flowers. It consists of a cylindrical vessel, C, of tin plate, provided on the bottom with the stop-cock a and the pipe b. The lid D fits into a gutter, R, running around the edge of C, and is hermetically closed by water in R. The cylinder is filled with the vegetable substance to be extracted, and sufficient petroleum-ether or bisulphide of carbon to cover it, poured in. The lid is then adjusted, the gutter R filled with water and the apparatus allowed to stand quietly for forty minutes. To remove the fluid from the cylinder, the faucet o in the lid is first opened, and then the stop-cock a; the fluid escapes at b, and is caught in a well-closed vessel. The operation may be repeated once or twice, or the vegetable substance is pressed out by means of a wooden plate, and the apparatus filled anew. The faucet h serves for emptying the gutter R.
Fig. 11.
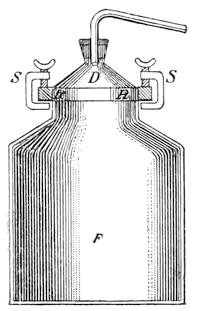
Extraction being finished, the cock o is opened, and then the cock a, and the fluid allowed to run into the flask of the distilling apparatus (Fig. 2). For working on a large scale, the flask is, however, too small, and is suitably replaced by a bottle-shaped tin vessel, F (Fig. 11), the conical cover D of which is secured by means of the rubber ring R and iron screw-clamps, S. A bent glass tube fitted into the cover is connected with the cooling-pipe of the apparatus shown in Fig. 2. But the oils prepared by extraction are not sufficiently purified by mere rectification, as traces of the solvent adhere tenaciously to them, which can only be removed by passing a current of air through the oil. But contact with air has an injurious effect upon the delicacy of the odor. For expensive oils a current of air should therefore never be used, but one of pure carbonic acid. Fig. 12 shows a suitable apparatus for the purpose. The large bottle A, filled half full with pieces of white marble, is closed with a doubly-perforated cork; through one of the holes is inserted a funnel-tube, and through the other a short tube bent at a right angle. The latter is connected with another tube which reaches to the bottom of the vessel B, in which is also inserted a tube open in the bottom, and a short tube bent at a right angle. Alongside B stands another vessel, C, arranged in the same manner. The tube leading from C is connected with a tin pipe, D, with a rose-like expansion on its lower end. This pipe is inserted in the glass balloon containing the volatile oil. Finally, a pipe leads to the flask F, filled with water.
Fig. 12.
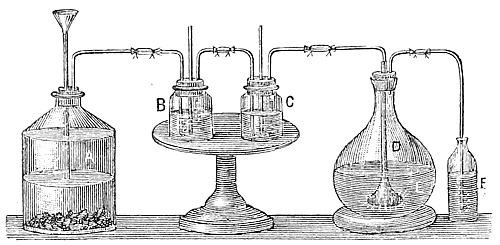
To put the apparatus in operation, strongly diluted hydrochloric acid is poured through the funnel-tube upon the pieces of marble in A, which causes the development of a current of carbonic acid. But as the latter carries along water and hydrochloric acid, it has to be freed from them before coming in contact with the volatile oil. The vessels B and C serve for the purpose. B is half filled with water, while C contains strong sulphuric acid. In B the hydrochloric acid carried along with the current of carbonic acid is retained, while the water is fixed on the sulphuric acid in C. The current of carbonic acid passing out from C is perfectly pure, and enters the volatile oil through the fine perforations in the pipe D. It absorbs the traces of solvent still adhering to the oil, and finally passes out through the water in the bottle F.
Volatile oils obtained by extraction, and purified by a current of carbonic acid, will keep for years without undergoing alteration, if placed immediately in hermetically closed vessels and stored in a dark place. Oils purified by a current of air always become somewhat thickly fluid by storing, and partially lose their fine odor, which is due to the oxygen absorbed during the process.
For the extraction of oil on a larger scale, the apparatus shown in Fig. 13 is very suitable. It consists of two principal parts, the actual extracting vessel E, and the still B. The extracting vessel E sits in a vat containing cold water, W, the arrangement being such that the heated water can be removed and replaced by cold. The still B sits in a boiler, K, filled with hot water.
The apparatus is charged as follows: The conical head C of the extracting vessel E is unscrewed and its connection at H with the pipe R loosened. The extracting vessel is then charged with the vegetable substance, the head C replaced, and the connection with the pipe R restored. The cocks H2 and H4 are then opened, and the required quantity of solvent is brought into the still. Both cocks are then closed, and the cocks H and H1 opened. The water in the boiler is then heated until the contents of the still commence to boil. The vapor of the solvent ascends through the pipe R; on entering the extracting vessel E it is condensed, and after falling as a spray upon the material to be extracted, finally returns impregnated with volatile oil to the still B. Here the solvent is revaporized, and passes again through the material in the extracting vessel, while the extracted oil remains in the still. During the boiling of the solvent the extracting vessel must be suitably cooled by the constant admission of cold water.
Fig. 13.
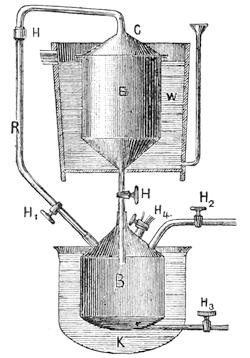
When extraction is finished, the cocks H and H1 are closed, and the cock H2, which is connected with a cooling worm, is opened. The solvent is then evaporated, and regained by condensation. The oil is discharged, from the still through a pipe in the bottom provided with the cock H3.
The apparatus may also be so arranged that the still B is connected with two extracting vessels which are used alternately, while the contents of one are being extracted the other is emptied and refilled.
Fig. 14.
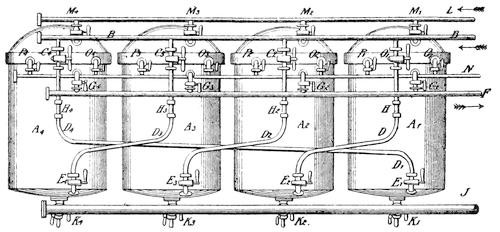
For working on a very large scale, Heyl's extracting apparatus, shown in Fig. 14, is very suitable. It consists of a battery of four or more cast iron or sheet iron cylinders, A1 to A4, communicating with each other and surrounded by steam jackets. The extracting vessels are so arranged that they can be emptied by tilting, which is rather inconvenient, as all the pipes have to be unscrewed. In each cylinder close above the bottom is a perforated plate covered with fine wire-gauze, upon which the material to be extracted is placed. The cylinder is filled to the top, and, after placing a similar plate upon it, the upper opening is closed by a lid suspended to a crane. The cylinder, as well as the lid, is provided with a broad flange, between which is placed a hemp tissue firmly pressed together by 12 clamps to serve for packing. After filling the cylinders with the material to be extracted and arranging the packing, the solvent (bisulphide of carbon) is conducted from a reservoir through the principal pipe, B, to the extracting vessels, and is introduced into A2 by opening the cock C2, which communicates with the pipe B. The bisulphide of carbon passes through the bent pipe D1, enters through the cock E2, below the false bottom of the cylinder A2, and, after penetrating the mass and filling the cylinder, runs through the cock C2 of the bent pipe D2, and the cock E3 into the cylinder A3, reaching the fourth cylinder in the same manner through the cock C3, the pipe D3, and the cock E4. From the last cylinder it passes as a thoroughly saturated oil solution into a reservoir, in which a vacuum has been created to promote the circulation of the fluid in the entire apparatus. After a quantity of oil solution corresponding to the contents of the cylinder A4 has arrived, the cock G4 is closed and the cock C4 opened, whereby the cylinder A4 is connected with A1 by the bent pipe D4 and the cock E1.
After the exhaustion of the contents of the cylinder A2, which is recognized by means of the glass tube H2 placed on D2 by the fluid running off being colorless, the cocks C1 and E2 are closed, and C2 and E3 opened, whereby the solvent runs into A3, and from there to A4 and A1; A2 being omitted. To effect this omission, and at the same time not to prevent the introduction of bisulphide of carbon, C1, C2, C3, and C4, are so-called two-way cocks, which, when placed in one position, connect the principal pipe B with the branch pipes D, but interrupt a further flow through the principal pipe B; while in the other position they close the pipes D and open the principal pipe B.
The cylinder A2 is, however, still filled with the solvent and material saturated with it. To remove the solvent, the discharge cock K2 on the bottom of the cylinder is opened, which communicates with the discharge pipe J, through which the bisulphide of carbon is conducted into a reservoir. The discharge is promoted by opening the cock M2, connected with the pipe L, and the admittance of compressed air, which displaces the liquid solvent. After the flow of the latter has ceased, the steam cocks on the jacket O2 and the cylinder P2 are opened under constant admission of air and simultaneous introduction of steam through the pipe N into the upper part of the cylinder.
The solvent (bisulphide of carbon) converted into vapor by the heat, is conducted together with the aqueous vapor, by the admission of air through the cock K2, the pipe J, and a cooling pipe placed between the extracting vessels and the reservoir, and collected in a reservoir to be re-used.
On account of the great volatility of bisulphide of carbon, considerable loss would, however, be incurred by the above-mentioned admission of air. To avoid this, the reservoir serving for the reception of the condensed bisulphide of carbon and aqueous vapor is closed, and connected by a pipe with a long, narrow, horizontal cylinder half filled with oil, and provided with a fan-shaft. The vapors of bisulphide of carbon entering the cylinder from the reservoir are absorbed, together with the air by the oil, the surface of which is constantly agitated by the fan-shaft, while the air, rendered entirely inodorous, passes out at the other end. The bisulphide of carbon is finally separated from the oil by distillation and again used.
After the cylinder A2 is sufficiently steamed, it is emptied and again charged with material and connected with the cylinder A1; while the other cylinders undergo the same manipulations described above.
Fig. 15.
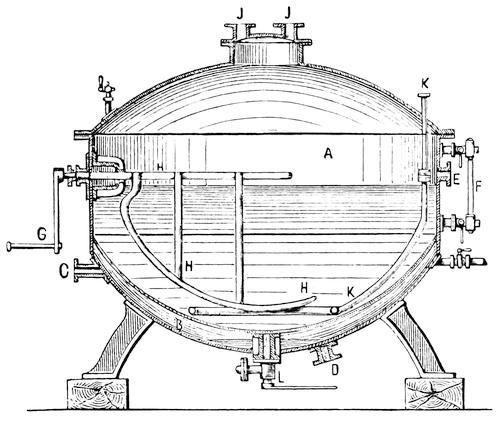
The saturated oil solution is subjected to distillation, which is readily effected in Heyl's apparatus, Fig. 15. The lower part of the still A of boiler plate is surrounded by the steam-jacket B, into which steam is admitted through C and the condensed water discharged through D. The concentrated oil solution runs from a reservoir, standing at a higher level through the pipe E into the still, the admission of a sufficient quantity being indicated by the gauge F. The bisulphide of carbon brought to the boiling point (114° F.) by the steam introduced into the jacket, vaporizes quickly; the vaporization being still more accelerated by revolving the stirrer H, by means of the crank G. The vapors of bisulphide of carbon escape through four openings in the upper part of the still, into a capacious worm, the lower part of which enters, under water, a reservoir.
Notwithstanding the volatility of bisulphide of carbon, the oil retains a portion of it so tenaciously that a complete separation cannot be accomplished by the introduction of steam into the jacket B. Hence, in order to vaporize the last traces of the solvent, air is introduced into the oil through the pipe K, the lower end of which is perforated. After completed distillation the oil is discharged through L.
Maceration or infusion.—This process is employed for flowers with an inconsiderable content of volatile oil or whose odoriferous substance would suffer decomposition or alteration by distillation. The process is founded on the affinity of odoriferous substances for fatty bodies which, when impregnated with them, are called pomades. These are afterwards made to yield the aroma to strong alcohol, so that finally there is obtained a solution of the volatile oil in alcohol from which the pure oil is obtained by distilling off the alcohol. The fat used, olive oil, lard, etc., should be entirely neutral, i. e., free from every trace of acid. The fats are purified by treating them several times in the heat with weak soda-lye and then washing carefully with water until the last traces of the lye are removed, and the fat shows no alkaline or acid reaction.
With the use of olive oil the so-called "Huiles antiques" are obtained, which are merely solutions of volatile oils in the fixed oil. By the use of lard, etc., the genuine pomades are obtained, which are directly used as expensive articles of perfumery, but in the factories serve as a starting point for the preparation of volatile oils.
The old process of maceration, which is still in use in some parts of France, is as follows: A certain quantity of fat is placed in an enameled iron or porcelain pan provided with a water or steam bath. When the fat is melted, the freshly gathered flowers from which the aroma is to be extracted are thrown in and left to digest for from twelve to twenty-four hours, the fat being kept fluid and stirred frequently. When the flowers are completely exhausted, the fat is strained from them into fresh pots, in which it is again macerated with fresh flowers as before. This operation is repeated ten to fifteen times until the pomade has acquired the desired strength.
Experience, however, has shown that volatile oils prepared by this process possess a finer odor the shorter the time the flowers remain in contact with the fat. Piver has devised an apparatus which reduces the time of maceration to the shortest period possible. The kettle to the left, Fig. 16, supplies the fat heated to the proper temperature, which circulates slowly through the macerating tank, in which a constant temperature of 149° F. is maintained by means of a steam pipe. The macerating tank is divided into compartments, in which baskets containing the vegetable substance to be extracted are suspended. The basket on the left contains the substance which has passed through all the compartments; it is from time to time removed, filled with fresh substance, and then attached to the right, the other baskets being moved to the next compartment to the left. In this way the fresh substance has to traverse each compartment from right to left, while the fat flows slowly from left to right, and saturated with the perfume of the substance collects in the tank on the extreme right.
Fig. 16.
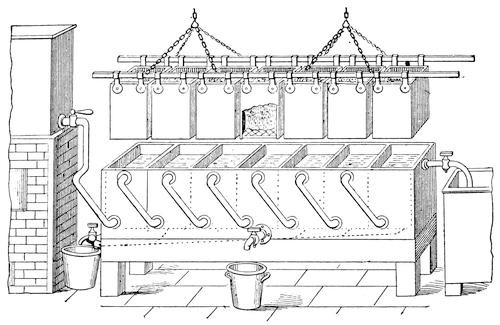
Maceration is employed for the flowers of the orange (citrus aurantum), of the mock orange (Philadelphus coronarius), of the acacia (acacia Farnesiana), of the violet (viola odorata), of the mignonette (réséda odorata), etc.
The process of absorption, or "enfleurage," as it is called by the French, is chiefly made use of for procuring the odoriferous principle of very delicate flowers, the delicious odor of which would be greatly modified, if not entirely spoiled, by the application of heat. The older apparatus employed for the purpose consists of a number of shallow wooden frames of about 15×18 inches, inclosing at half their depth a sheet of glass. The edges of the frame rise about an inch above each surface of the glass, and, being flat, the frames stand securely upon one another, forming often considerable stacks. These frames are called "chassis," those just described being termed "chassis aux vitres," or "chassis aux pomades," to distinguish them from a different form, which is used where oil has to be submitted to the process of absorption. The process in the case of pomade is as follows: Each sheet of glass is uniformly coated with a thin layer of purified grease, care being taken that the grease does not come in contact with the woodwork of the frames. The flowers are then thinly sprinkled, or rather laid, one by one, upon the surface of the fat, where they are allowed to remain one or two days, when they are removed and replaced by fresh ones. The operation is thus continued for twenty-five or thirty days, until the fat is saturated with aroma. The frames charged with fat and flowers are stacked one upon the other, forming, in fact, a number of little rectangular chambers.
For perfuming oils a metal sieve, Fig. 17, is substituted for the glass plate. Upon the sieve a piece of thick cotton cloth saturated with oil is laid, and upon this the flowers are scattered, and left there until fresh ones have to be substituted. The operation is repeated until the oil is sufficiently impregnated with aroma, when the cloth is subjected to pressure and the expressed oil filtered.
Fig. 17.
This process is very tedious, requiring much labor and a long time for the impregnation of the fat or oil, but, notwithstanding its faults, it is still pursued to a great extent, some French firms using 3000 such frames during the season.
With the apparatus, shown in Fig. 18, the process of absorption can, however, be conducted with very little expense of labor and time. It has the further advantage that the flowers do not come in direct contact with the fat, whereby a saving of the latter is effected, and it is less liable to rancidity.
The apparatus consists of a tall wooden box provided with doors which can be hermetically closed. In the box are placed upon brackets a number of glass plates, g, so arranged one above the other that, for instance, those with uneven numbers are on the left side, leaving an open space to the right, while those with even numbers are arranged on the right side with an open space to the left.
From the bottom of the box a pipe passes into a sheet-iron cylinder, K´, filled loosely with flowers, and provided with lateral openings, O and O´. From the lid of the box K ascends a pipe, e, which is connected with a small ventilating apparatus kept in motion by a clockwork and weights. This ventilator when in motion sucks a current of air through the apparatus. The air enters the cylinder K´ at O, and after ascending through the flowers and becoming impregnated with the vapors of the volatile oil enters through the opening O´ into the box K and, in passing in the direction indicated by arrows, over the plates coated with fat, yields its aroma to them.
Fig. 18.
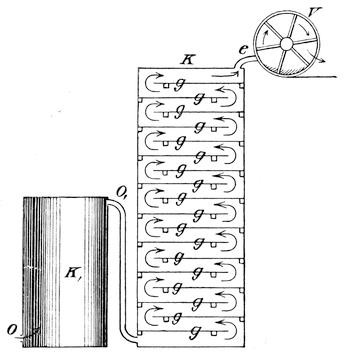
Another apparatus for the same purpose, devised by Piver, is shown in Fig. 19. The fat is converted into thin macaroni-like threads and brought upon wire gauze stretched in frames. The flowers to be extracted are piled upon tinned metallic plates, and the trays containing the fat and the flowers are placed in an air-tight chamber arranged as shown in the illustration. The air in the chamber is made to circulate to and fro by the working of a bellows with which the apparatus is provided, whereby the fat is caused to absorb the odor of the flowers very rapidly and is less liable to rancidity.
Fig. 19.
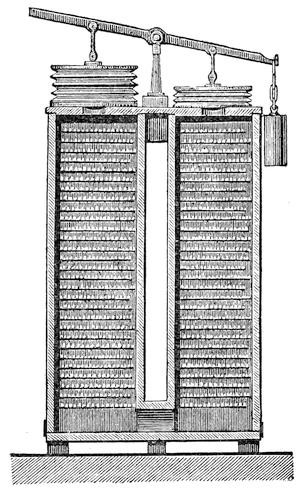
The absorption process is employed for the flowers of the jasmine (jasminum oderatissimum), the mignonnette (réséda odorata), the violet (viola tricolor), the tuberose (polianthes tuberosa), etc.
Storage of volatile oils.—In storing volatile oils, they should be carefully protected from light and air. Some oils become darker on exposure to light, while others, for instance, lemon oil, become colorless. Most volatile oils, as previously mentioned, absorb oxygen from the air with avidity and combine chemically with it. Thinly-fluid oils become perceptibly more thickly-fluid and finally even rigid, the product of oxidation being a resinous body. Some volatile oils containing aldehydes are converted, by the absorption of oxygen, into acids, cinnamic acid being, for instance, formed in cinnamon oil, and benzoic acid in oil of bitter almonds.
To prevent evaporation, as well as the above-mentioned effects of light and air, the volatile oils should be preserved in not too large glass bottles kept as full as possible, and closed with a good cork, over which it is best to tie a piece of bladder. The bottles should be stored in a cool, shady place. The preservation of the oils is assisted by the addition of 0.5 to 1 per cent. of anhydrous alcohol.
CHAPTER III.
TESTING VOLATILE OILS.
Volatile oils are much adulterated, the adulterations consisting chiefly in mixing an expensive oil with a cheaper one and with alcohol; more rarely with chloroform and fat oils. To these adulterations, which have been common for many years, has recently been added the previously mentioned hydrocarbon called terpene or camphene, which is separated in the preparation of concentrated oils.
For the recognition of the quality of a volatile oil, serve first of all its physical properties, especially its color, odor and taste. The specific gravity varies too much and is not always a sufficient criterion. Reagents can only be employed with a few oils. The chemical detection of adulterations is rendered especially difficult by the fact, that most of the volatile oils form a mixture of terpenes with other combinations, in which the separate constituent parts do not appear in fixed, but in changeable proportions, and in which the constituents themselves suffer alteration by storing, air and light.
Odor and taste are so characteristic for every volatile oil as to suffice in most cases. For testing as to odor, bring a drop of the oil to be examined upon the dry palm of one hand and for some time rub with the other, whereby the odor is more perceptibly brought out. To determine the taste, vigorously shake one drop of the oil with 15 to 20 grammes of distilled water and then test with the tongue.
An adulteration with fat oil (poppy oil, castor oil) may be recognized as follows: Place a drop of the suspected oil upon blotting paper and expose it to the heat of the water bath. If it evaporates completely and no stain is perceptible, the oil is pure. But frequently a transparent stain remains with old oils without their being adulterated, which is due to the resin formed by the absorption of oxygen and remaining dissolved in the oil. In this case a transparent ring is generally formed by the concentration of the resin on the edges of the stain. If no tangible results are obtained by this test, pour a few cubic centimeters of the oil upon a watch-crystal and heat it very slowly upon a piece of sheet-iron, until all the odor has disappeared. If the watch-crystal becomes empty in a short time, nothing but volatile oil was present; but if a viscous residue remains, this may consist either of fatty oil or resin, or of both. Treat the residue with strong alcohol; if it dissolves it may be resin or castor oil. Dilute the solution with much water; a white flocculent turbidity indicates resin; the separation of an oily liquid, after standing, castor oil. If the residue remains undissolved, it consists of a fatty oil, generally oil of almond or olive.
The presence of castor oil can be accurately determined by bringing the residue from the watch-crystal into a test-tube by means of a glass-rod, and compounding it with a few drops of nitric acid. A strong development of gas takes place, after the cessation of which, solution of carbonate of soda is added as long as there is any sign of effervescence. If the added oil was castor oil, the contents of the test-tube will show a peculiar odor due to œnanthylic acid formed by the action of nitric acid upon castor oil.
Another method of establishing the presence of fat oil consists in mixing the suspected oil with eight times its quantity of 90 per cent. alcohol (specific gravity 0.823). If the oil is unadulterated a clear solution is formed; if it contains fat oil, the latter remains undissolved. The presence of castor oil, which of the fat oils is chiefly used for adulteration, is, however, not shown by this method, it being also soluble in alcohol.
A permanent stain upon the paper may, however, also be formed by fresh oils obtained by expression from the respective parts of the plant. Thus, lemon oil obtained by expression from the peel, and which has a far more agreeable odor than that produced by distillation, always leaves behind a slight grease-stain.
Detection of alcohol or spirit of wine.—Independent of the alcohol added to assist the preservation of some oils, adulteration with alcohol frequently occurs, especially in expensive oils. With a content of not more than 3 per cent. of alcohol, it suffices to allow one to two drops of the suspected oil to fall into water. In the presence of alcohol, the drop becomes either immediately surrounded with a milky zone, or it becomes turbid or whitish after being for some time in contact with the water. Dragendorff's test is based upon the fact that oils, which are hydrocarbons, suffer no change by the addition of sodium (ten drops of oil and a small chip of sodium), while oils containing hydrocarbons and oxygenated oils cause with sodium a slight evolution of hydrogen gas, and suffer but a slight change during the first five to ten minutes of the reaction. If, however, the oil is adulterated with alcohol, not only a violent evolution of hydrogen gas takes place, but the oil in a short time becomes brown or dark brown, thickly fluid or rigid.
The detection of alcohol by means of fuchsine, which has been frequently recommended, requires special precautions. It must first be ascertained that the oil is free from acids and water; if such is not the case, they must be removed by means of caustic potash. After settling, bring, by means of a dry pipette, about five cubic centimeters of the oil into a dry test-tube about ten millimeters in diameter, without moistening the walls of the upper half of the tube. Then bring, by means of a paper gutter, a few milligrammes of coarsely-powdered fuchsine into the dry part of the obliquely held tube, at a distance of one centimeter from the oil. Now heat gradually over a lamp until the tube begins to tarnish. With pure oil no evaporation is observed, but if the oil contains only 0.1 per cent. of alcohol, every speck of fuchsine will, after heating to boiling and setting aside, be surrounded by a stain produced by the alcoholic solution. The chief requirement for this test is that the oil be free from water. If such is not the case, vapors will be observed, which condense in the upper portion of the test-tube, and dissolve fuchsine, and, after flowing back, sink below the oil with a crackling noise. If the oil contains alcohol, the condensing vapors dissolve fuchsine with greater ease, and in flowing back mix without crackling.
Hager's tannin test is very reliable. Bring into a test-tube 5 to 10 drops of the oil to be examined, add a piece of tannin the size of a pea, shake so that the tannin is moistened by the oil, and let the whole stand at a temperature of 59° to 68° F. In most volatile oils tannin is insoluble, and, if the oil is pure, floats for days on the surface without change. If, however, the oil contains alcohol, the tannin absorbs the latter, according to the quantity present, in 3 to 48 hours, and forms with it a more or less transparent, viscous, tough, or smeary mass resembling a soft resin, which settles on the bottom, and adheres so firmly to it, as well as to the sides of the tube, that it cannot be moved by shaking. The mass may be examined as to its consistency with a knitting needle. Traces of moisture in the oil are not detrimental to the test, the tannin mass separating in the form of a hyaline mass only in few oils, and if this mass is tested with the knitting needle it will be found not tough or smeary, but hard, and may sometimes be divided into small grains. With oil of bitter almonds, cassia oil, and some oils of clove, as well as volatile oil containing an acid, the tannin test is not available. The first two oils even dissolve tannin, and large quantities of it, if they contain alcohol.
The above-mentioned oils may, however, be rendered fit for the tannin test by mixing them with double their volume of benzine or petroleum-ether, and allowing the mixture to stand for two or three days. If, however, the oils contain much alcohol, the tannin is dissolved. The use of powdered tannin is not advisable, because it generally deposits in a thin layer on the bottom, and its alteration is not so perceptible. If, for practical reasons, a content of 0.5 per cent. anhydrous alcohol might be accepted as permissible in a volatile oil, the tannin test would have to be so modified as to mix 10 drops of the oil with a piece of tannin the size of two peas, and allow the whole to stand for one hour. In this time the above-mentioned content of alcohol would yield no result.
Detection of chloroform.—An adulteration with chloroform, if moderate, cannot always be detected by the odor and taste. In most cases, chloroform will considerably increase the specific gravity of the oil. Bring into a test-tube 15 drops of the suspected oil, 45 to 90 drops of alcohol, and 30 to 40 drops of dilute sulphuric acid. After thorough shaking, add 2 or 3 shavings of zinc sheet and heat until a vigorous evolution of hydrogen takes place. After again shaking, set the whole aside, and heat again when the evolution of gas becomes weaker. This heating and gentle shaking of the fluid is several times repeated. After 20 to 25 minutes, compound the fluid with an equal volume of cold distilled water, shake vigorously and filter through a paper-filter moistened with water. Strongly acidulate the filtrate with nitric acid and compound with nitrate of silver solution. If chloroform is present, turbidity or a precipitate of chloride of silver appears.
Detection of benzine.—An adulteration with benzine can be readily detected only in oils specifically heavier than water. The separation of benzine is effected by distillation from a small glass flask in the water bath. The distillate together with an equal volume of nitric acid of 1.5 specific gravity is gently heated in a test-tube. A too vigorous reaction is modified by cooling in cold water, and a too sluggish action quickened by gentle heating (dipping in warm water). If the mixture has a yellow color, dilute it with water, shake with ether, mix the decanted ethereal solution with alcohol and hydrochloric acid, add some zinc and place the whole in a lukewarm place to convert the nitrobenzol formed into aniline. After evolution of hydrogen is done, neutralize with potash lye, shake, take off the layer of ether, let the latter evaporate and add to the residue a few drops of calcium chloride solution. If benzine is present, a blue-violet color reaction takes place.
Adulterations with alcohol, chloroform, and benzine are quantitatively determined by bringing a weighed quantity of the oil into a glass flask so that it occupies about four-fifths of the volume of the flask. Place upon the flask a cork through which has been passed a glass-tube bent at a right angle and provided with a cylindrical glass vessel serving as a receiver and heating in the water bath. If the distance from the level of the oil to the angle of the glass tube in which it inclines downwards, amounts, for instance, to 4.72 inches, and the neck of the flask up to its angle is 2.75 inches high outside of the direct effect of the heat of the water bath, only the above-mentioned adulterants distill over, while the vapor of the volatile oil condenses at a height of 2.75 inches and flows back into the flask. The distillate is weighed and examined as to its derivation. First add one cubic centimeter of it to two or three cubic centimeters of potassium acetate solution of specific gravity 1.197 and shake moderately. If a clear mixture results, alcohol alone is present. If, however, the mixture is not clear, and the distilled fluid sinks down and collects on the bottom of the test-tube, chloroform is very likely present, and if it remains floating upon the acetate solution, benzine. Next bring two to three centimeters of the distillate into a test-tube and add a piece of sodium metal, the size of a pea. If violent foaming, i. e., an evolution of gas, takes place, alcohol is certainly present, and possibly also chloroform and benzine towards which sodium is indifferent. However, in the presence of benzine, the sodium solution would be colorless, and in the presence of chloroform, yellowish and turbid. In case the sodium produces no reaction and alcohol is, therefore, not present, add an equal volume (two to three cubic centimeters) of anhydrous alcohol, and after moderately shaking allow the solution of the sodium and the evolution of gas to proceed, whereby benzine produces a nearly colorless, turbid fluid, and chloroform a yellowish, milky one. Now dilute the fluid with an equal or double volume of water, shake and allow the mixture to stand quietly. In the presence of benzine a colorless, turbid layer collects on the bottom of the fluid, while that collecting in the presence of chloroform is yellowish. In the latter case, i. e., in the presence of chloroform, the aqueous filtrate yields with lead acetate solution a white precipitate (lead chloride and lead hydroxide). The adulterant having thus been recognized, further particulars are learned from the specific gravity of the oil as well as of the distillate.
Adulterations with terpenes or terpene-like fluids, such as are gained in the preparation of concentrated or patent oils, are difficult to recognize. They may be detected by the specific gravity, the terpenes being, as a rule, specifically lighter, their specific gravity varying between 0.840 and 0.870.
The detection of adulterations with volatile oils of a lower quality is very difficult, if not led to it by the odor and taste. Many methods for establishing such adulterations have been proposed, of which the following are the most important:—
I. Test with iodine.—This test is based upon the fact that some oils violently detonate with iodine, while others develop heat and vapors, and others again remain indifferent. For this test pour upon about 0.19 gramme of dry iodine in a watch-crystal 4 to 6 drops of the oil to be examined.
1. A vigorous reaction (detonation) with considerable increase in the temperature and emission of vapors takes place with the following oils: oils of bergamot, lemon, lavender, nutmeg, orange peel, spike, turpentine, wormwood.
2. Such a reaction as mentioned under 1, does not take place with oils of bitter almonds, copaiba, calamus, clove, peppermint, rose.
3. Moderate heating and slight vapors are developed with oils of anise-seed, fennel, camomile, curly mint, marjoram, rosemary, sassafras, thyme.
When an oil of the second series becomes heated with iodine and evolves vapors, it may first of all be adulterated with cheaper oils. This may also be the case when an oil of the third series reacts violently with iodine and evolves vapors with strong heating. Formerly the iodine test was highly valued; it has, however, been shown to be unreliable since it is frequently dependent on the age of the oil.
In place of iodine, Rudolph Eck recommends a very dilute alcoholic iodine solution, which is not discolored by oils of turpentine, while other oils discolor it. Dissolve a drop of the oil to be examined in 3 cubic centimeters of 90 to 100 per cent. alcohol, and add a drop of the iodine solution. The latter is not discolored in the presence of an oil of turpentine. There are also, however, several volatile oils, which do not discolor the iodine solution. Mierzinski mentions the following: All cold-expressed oils from the Aurantiaceæ, further oils of coriander, caraway, galanga, rue, sassafras, rose, rosemary, anise-seed, fennel, calamus, neroli, angelica, wormwood. Hence, this reaction cannot be relied upon.
II. Hoppe's nitroprusside of copper test.—This test sometimes gives good results, but only with hydrocarbons absolutely free from oxygen and oxygenated oils. It is, therefore, not suitable for oils derived from the Aurantiaceæ. The process is as follows: Add to a small quantity of the oil to be examined in a perfectly dry test-tube, 2 to 5 milligrammes of pure nitroprusside of copper previously thoroughly dried and finely pulverized, shake vigorously and gradually heat to boiling. After boiling for a few seconds allow to cool. If the oil is free from oil of turpentine, or another oil containing no oxygen, the precipitate formed is brown, black, or gray, and according to the quantity of the reagent added and the original color of the oil, the supernatant oil will be differently colored and appear more or less dark. If, however, the oil is adulterated with oil of turpentine, the precipitate formed shows a handsome green or blue-green color, while the supernatant oil retains its original color or at the utmost acquires a very slightly darker one. The longer the oil is allowed to stand after settling, the more distinct and beautiful the color of the oil and of the precipitate appears. For the establishment and certain recognition of very small quantities of oil of turpentine in oxygenated oils, it is best to first add very little of the nitroprusside of copper to the oil to be tested, and a larger quantity only after being convinced either of the purity or adulteration of the oil. This is done to be able, on the one hand, better to judge the reaction, if the oil is pure, and, on the other, if it is adulterated, to establish such adulteration with certainty and to approximately estimate the quantity of oil of turpentine present. The less nitroprusside of copper is used, the better small quantities of oil of turpentine can be detected.
Nearly all volatile oils free from oxygen show the same behavior towards nitroprusside of copper; they decompose it, which is not the case with oxygenated oils. The behavior of the latter is shown in the following table:—
Name of the oil
Color of the oil
Proportion of nitroprusside of copper to oil
Color of the oil after the experiment
Color of the Precipitate
Caraway
clear as water and colorless
1:1000 parts
slightly yellowish
dirty gray.
Fennel
pale yellowish
1:1000 "
brownish-yellow
black.
Dill
pale reddish-yellow
1:1000 "
becomes first colorless, then yellowish
"
Anise-seed
pale yellow
1:1000 "
yellow
"
Camomile (green)
yellowish
1:1000 "
brownish-yellow
ash-gray.
Lavender
pale yellow
1:1000 "
wine-yellow
slate-gray.
"
"
1: 100 "
brown-yellow
"
Mint(curly)
colorless
1:1000 "
wine-yellow
first gray, then black.
Peppermint
"
1:1000 "
yellowish
black.
"
"
1: 100 "
brownish-yellow
"
Balm
yellow
1:1000 "
dark wine-yellow
"
Marjoram
colorless
1:1000 "
yellowish
"
"
"
1: 100 "
brown-yellow
"
Sage
slightly yellowish
1:1000 "
wine-yellow
dark green.
"
"
1: 100 "
brown-yellow
dark green, then nearly black.
Thyme(field)
"
1:1000 "
brownish-yellow
slate-gray.
"
"
1: 100 "
darkbrown-yellow
nearly black.
Wormwood
yellow-brown
1:1000 "
dark brown
black.
Tansy
pale yellow
1:1000 "
red-brown
dirty brown.
Milfoil
dark azure-blue
1:1000 "
first pale blue, then dark green
gray-brown.
Cajeput
colorless
1:1000 "
brownish-yellow
black.
Clove
slightly yellowish
1:2000 "
rose-red and clear
slate-gray.
"
"
1:1000 "
violet-red and clear
"
"
"
1: 500 "
cherry-red and opaque
"
"
"
1: 100 "
dark cherry-red and opaque
"
Cassia
brownish-yellow
1:1000 "
brownish-red to hyacinth-red
black.
"
"
1: 100 "
dark brown-red
"
Sassafras
yellowish
1:1000 "
yellowish-brown
"
Star anise
pale yellow
1:1000 "
dark wine-yellow
"
Valerian
pale greenish
1: 100 "
brownish-yellow
"
Rue
slightly yellowish
1: 100 "
brown-yellow
ash-gray.
Bergamotte
yellowish
1:1000 "
dark yellow
"
"
"
1: 100 "
brownish-red
"
If these oxygenated oils are mixed with oils free from oxygen, for instance, oil of turpentine, they show exactly the same behavior as oils free from oxygen; the nitroprusside of copper is not decomposed and retains its gray-green color. If, for instance, oil of cloves is mixed with oil of turpentine, the red coloration by nitroprusside of copper does not appear.
III. Hager's alcohol and sulphuric acid test.—Bring into a test-tube of about 0.5 inch diameter, five to six drops of the oil to be tested and twenty-five to thirty drops of pure concentrated sulphuric acid, and mix the two fluids by shaking, whereby either no heating takes place or a scarcely perceptible one, or the heating is strong or very vigorous and in some cases increased to the evolution of vapors. The mixture is either clear or turbid. After complete cooling, add to the mixture eight to ten cubic centimeters of 90 per cent. alcohol, and after closing the tube with the finger, shake vigorously. The mixture now shows a different color, is clear or turbid, and the deposit formed after standing for one day is also differently colored and either soluble or insoluble in boiling alcohol.
The mixture of oil, sulphuric acid and alcohol is perfectly clear and transparent with oils of bitter almonds, fennel, clove and rose; with anise-seed oil and star anise-seed oil only the alcoholic layer over the mixture of sulphuric acid and oil is clear. The mixture of oil, acid and alcohol is slightly turbid or nearly clear with oils of valerian, peppermint and field thyme. With most of the other volatile oils occurring in commerce, the mixture is more or less milky turbid. Heating of the oil and acid mixtures does not take place with pyrogenous oils (petroleum, benzine) or only to a very slight degree, as with oils of peppermint and mustard.
IV. Hager's guaiacum reaction[3] serves for the detection of oil of turpentine in a volatile oil. By pouring upon as much guaiacum, freshly powdered, as will lie upon the point of a small knife, in a test-tube 1 cubic centimeter (25 drops) of spike oil, and heating nearly to boiling over a petroleum lamp, the oil after being removed from the flame and allowing the undissolved resin to settle, shows a yellow color. By now pouring upon an equal quantity of guaiacum in another test-tube 25 drops of spike oil and 5 drops of rectified oil of t from the flame shows a dark violet color. Various other oils behave in the same manner as spike oil, and hence a content of oil of turpentine can be readily detected in them. Other oils do not exhibit this behavior; but this can be remedied by adding, in testing for oil of turpentine, a few drops of an oil of the first class.
The guaiacum reaction is an ozone reaction and with reference to this, the volatile oils may be divided into three classes:—
a. Oils inclining to the formation of ozone.—Foremost of these is oil of turpentine, especially when rectified. Oils of tansy, rue, mint, juniper, zedoary, etc., show considerably less inclination.
b. Oils which, especially when heated, directly incite the oil of turpentine to form ozone, and to color guaiacum violet or blue.—Such oils are many kinds of oil of citronella, oils of spike, calamus, cedar, etc.
c. Oils with a content of oil of turpentine, which remain indifferent towards guaiacum.—To such oils, if to be tested for oil of turpentine, with the assistance of the guaiacum reaction, a few drops of an oil of the second class have to be added.
V. Hübl's iodine method.—Mr. C. Barenthin has applied Hübl's iodine method for fixed oils to the examination of volatile oils. He uses the following solutions:—
1. Fifty grammes iodine and 60 grammes of mercuric chloride in a liter of alcohol freed from fusel oil, and let stand for 12 hours.
2. Twenty-four grammes of hyposulphite of sodium in a liter of water.
3. A ten per cent. solution of iodide of potassium. Dissolve 0.1 to 0.2 gramme of the volatile oil in 10 cubic centimeters of chloroform, and add first 15 cubic centimeters of the iodine-mercuric chloride solution; let stand three or four hours, and, in case the mixture gets discolored, add a few more centimeters of solution. Now add 10 to 15 cubic centimeters iodide of potassium solution, dilute with 150 cubic centimeters of water, and titrate with hyposulphite till the mixture remains clear for about a minute. The iodide of potassium solution must be added before the water, and the relative proportions between this solution and the iodine-mercuric chloride solution must be 15 to 20 cubic centimeters. The quantity of iodine solution consumed is calculated to iodine for 100 parts and the figure thus obtained is designated as the "iodine number."
Barenthin has in this manner determined the iodine number of several volatile oils; other experimenters, however, for instance, Kremel and Davies,[4] have found different numbers for the same oils, so that this method requires further thorough examination before it can be classed as available.
VI. A. Kremel has endeavored to utilize titration or saponification with alcoholic potash lye for the examination of volatile oils. In his experiments he was guided by the following points: A series of volatile oils contains partially free organic acids, like oils of bitter almonds and cinnamon, and partially aldehydes or other combinations. Now it seems not impossible, that up to a certain limit, the quantities of these combinations in the separate volatile oils remain constant, thus presenting the opportunity of testing the respective oils as to their quality and purity by saponification. In some cases these combinations are the chief bearers of the specific odor, and hence the determination of the "saponification number" becomes of double value. It is, of course, self-evident that not every volatile oil can be saponified, and Kremel admits that, even where saponification takes place, it is not in every case a sure test.
The execution of the method is as follows: Dissolve 1 gramme of the oil to be examined in 2 to 3 cubic centimeters of 90 per cent. alcohol freed from acid, compound the solution with a few drops of phenol-phthalein solution, and titrate the free acid with ½ normal alcoholic potash lye. The milligrammes of caustic potash used are designated the "acid number." After having thus determined the content of acid, add to the same solution 10 cubic centimeters of the same potash lye, heat for ¼ hour upon the water bath, and then titrate back the excess of potash lye with ½ normal hydrochloric acid. In this manner the "saponification number" is obtained. (In some cases when the final reaction is not plainly perceptible, it is advisable to correspondingly dilute with water after heating the alcoholic fluid.) The saponification number, less the acid number, gives the "ether or ester number."
Kremel has in this manner examined a large number of volatile oils and partially obtained surprising results. Rose oil gives a saponification number of 12, and geranium oils one of 40 to 50. While lavender oils give very high saponification numbers, oil of lemons does not. Artificial oil of bitter almonds shows higher saponification numbers than the natural oil. By further compounding the saponified portions of the latter with acid, a crystalline precipitate of benzoin is formed, the quantity of which amounts to from 40 to 50 per cent. of the oil used. Such a precipitate, but only in very small quantities, is also formed in peach kernel oil, but not in other similar oils nor in artificial oil of bitter almonds.
VII. F. R. Williams has recently endeavored to utilize for testing volatile oils Maumené's test, which is based upon the increase in temperature produced in oils by concentrated sulphuric acid, and which gives valuable points for the examination of some fat oils. Of course, the large quantities of oil otherwise prescribed cannot be used. While for the examination of fat oils 50 grammes of oil are mixed with 10 cubic centimeters of concentrated sulphuric acid in a beaker glass wrapped around with cotton, Williams could use only six cubic centimeters of volatile oil. They were brought into a very small beaker glass enveloped in cotton. After reading off the temperature, twelve cubic centimeters of concentrated sulphuric acid were added and the whole stirred with the thermometer until the temperature no longer rose. Numbers were in this manner obtained which might in some cases, for instance, cassia oil, furnish guiding points for judging the purity of the oil.
Planchon proposes the following procedure in order to recognize a volatile oil:—
A. The oil is specifically lighter than water.
1. The substance is solid and only melts at 347° F.: Camphor.
2. The oil at a temperature of over 32° F. contains a crystalline stearoptene.
a. The oil is laevorotatory, the stearoptene melts at 77° F., and, on adding sulphuric acid, a clear solution remains behind: Rose oil.
b. The oil possesses no rotatory power, the stearoptene melts at 50° F., and, on adding sulphuric acid, two layers are formed, only one of which is liquid: Anise-seed oil.
c. The oil is dextrorotatory, the stearoptene melts at 41° F., and, on adding sulphuric acid, a nearly colorless fluid remains behind: Fennel oil.
3. The oil is perfectly fluid and clear at above 32° F.
I. The oil explodes with iodine, emitting violet vapors.
a. The oil thickens in the air and readily forms resin.
It requires for its solution several volumes of alcohol: Oil of conifers.
b. The oil, on exposure to the air, does not thicken and but slowly forms resin.
α. It is dextrorotatory.
The liquid oil dissolves santalin: Oil of the aurantiaceæ.
The thick oil does not dissolve santalin: Mace oil.
β. The oil is laevorotatory.
The oil shows an acid reaction and dissolves in equal parts of alcohol: Lavender oil.
The oil shows a neutral reaction and dissolves in 12 to 15 parts of alcohol: Marjoram oil.
II. The oil gives no explosion with iodine, but shows an increase in temperature with or without emission of red vapors.
a. The oil shows an acid reaction.
α. The blue or green oil shows the acid reaction only indistinctly: Milfoil oil.
β. The colorless or brown oil gives a turbid fluid with sulphuric acid. It is laevorotatory: Spanish marjoram oil.
The oil is rendered but slightly turbid by sulphuric acid; it acquires a red-violet color by nitric acid, has no effect upon the plane of polarization, and has a peculiar odor: Oil of valerian.
b. The oil is neutral.
α. It dissolves with difficulty in alcohol.
β. The oil is miscible in every proportion with alcohol.
1. It is dextrorotatory.
The oil is colorless or yellowish, it thickens on exposure to the air, and dissolves and reduces fuchsine: Caraway oil.
The oil is thick, yellow-brown or red-yellow, and has a peculiar odor: Calamus oil.
2. The oil is laevorotatory.
It is fluid and has an aromatic odor: Rosemary oil.
The oil is thick and very pungent: Cubebs oil.
III. The oil dissolves iodine without vigorous reaction and without an increase in the temperature.
a. The oil is blue and green.
It has an agreeable, camphor-like odor: Camomile oil.
The green oil thickens in the air and is dextrorotatory: Wormwood oil.
The oil is generally green and produces no effect upon the plane of polarization: Cajeput oil.
b. The oil is colorless or yellow-brown.
α. It separates a solid stearoptene at about 32° F.: Rue oil.
β. The oil remains liquid at several degrees below 32° F.
1. Dextrorotatory oils.
The oil shows an acid reaction, and gives with sulphuric acid a somewhat turbid solution, which becomes clear by the addition of alcohol: Dill oil.
The oil gives with sulphuric acid a yellow-red turbid solution, which becomes clear and peach-blossom red by the addition of alcohol: Eucalyptus oil.
2. Laevorotatory oil.
The oil showing an acid reaction becomes thick in the air and has a characteristic odor: Mint oil.
The oil shows a neutral reaction and has a camphor-like odor: Thyme oil.
IV. The oil does not dissolve iodine, does not heat with sulphuric acid, and does not react upon nitric acid. The odor is empyreumatic: Petroleum.
B. The oil is specifically heavier than water.
1. The oil shows an acid reaction.
It is soluble in 30 parts of water, boils at 356° F., and smells of bitter almonds: Oil of bitter almonds.
The oil has an agreeable, sweet odor and boils at from 392° to 431.6° F.: Wintergreen oil.
2. The oil shows a neutral reaction.
a. The oil is laevorotatory.
It becomes blue by the addition of sulphuric acid: Oil of cloves.
b. The oil is optically inactive.
The thick oil gives with sulphuric acid a turbid, black-brown fluid; the odor is agreeable: Cinnamon oil.
c. The oil is dextrorotatory.
The thick oil has an agreeable odor: Sassafras oil.
[3] Hager, Chemische Reactionen zur Nachweise des Terpentinoels in den aetherischen Oelen, etc. Berlin, 1885.
[4] Pharm. Centralh. 1888, S. 482 u. 555; 1889, S. 133.
CHAPTER IV.
THE VOLATILE OILS USED IN PERFUMERY.
The volatile oils, as previously mentioned, may be divided into three groups, viz: the pure hydrocarbons, oxygenated oils, and sulphuretted oils. Chemically, this division is, however, of little value, since, among bodies which should be classed according to it in one of the groups, combinations are found which vary very much in a chemical respect, and belong partially in the groups of alcohols, indifferent bodies, acids, etc.
It is, therefore, preferred not to attempt a classification of the volatile oils according to their chemical composition, but simply to enumerate them in alphabetical order.
Acacia, oil of, commonly called oil of cassie. The flowers or buds of the acacia Farnesiana yield a somewhat thickly-fluid, greenish-yellow oil of a very intense but delightful odor. The oil may be obtained either by extraction or absorption. The acacia is cultivated in special plantations along the Riviera di Genova. These plantations being controlled by a few perfumers, the oil is not allowed to reach the market, and does not form an article of commerce. The green-colored extrait d'acacia is a solution of the oil in alcohol.
Almond oil (bitter) (oleum amygdalae amaræ) is obtained by submitting bitter almond cake (left after the expression of the fixed oil from bitter almonds) to distillation with water. The volatile oil does not exist ready formed in the bitter almond, nor in the almond cake, but results from the decomposition of a glucoside called "amygdalin," contained in the cake, under the influence of emulsin and water, the emulsin acting as a ferment, into benzylic aldehyde, glucose and prussic acid. The almond tree grows wild, but is also cultivated in Southern Europe, Africa, Barbary, Palestine and Syria. The bitter almonds brought from Barbary are considered the best. Besides, in almonds, amygdalin occurs in various other plants; for instance, in the leaves of the cherry laurel, the leaves and kernels of the peach, the kernels of the black cherry and other varieties of prunus and amygdalus, they all yielding, after maceration with water, a distillate containing prussic acid and oil of bitter almonds.
Instead of the comparatively expensive bitter almonds, peach kernels freed from their hard shells are extensively used in the fabrication of oil of bitter almonds. The oil is prepared as follows: The press cakes of bitter almonds or peach kernels are ground and soaked about twenty-four hours in twice their weight of water to which one-third their weight of salt has been added. The whole is then submitted to distillation. The temperature of the water should not exceed 113° to 122° F. The emulsin contained in the almonds possesses only within certain limits of temperature the power of decomposing amygdalin, and, if heated to 176° F., becomes inoperative. Hence, if the almond paste is quickly heated to boiling, the emulsin becomes inoperative before all the amygdalin is decomposed, and a portion of it being consequently lost, the yield is insufficient. The distillation of the almond paste is effected in a current of steam.
A portion of the prussic acid formed by the decomposition of the amygdalin adheres tenaciously to the oil. This content of prussic acid makes the oil of bitter almonds exceedingly poisonous, while in itself it is non-poisonous. It can be freed from the prussic acid by shaking with ferrous sulphate (blue vitriol) solution. By then distilling over burnt lime the originally yellow or yellowish oil is obtained colorless. It is then thinly fluid, of a peculiar agreeable odor and strongly nutty taste. Its specific gravity is 1.043 at 59° F., but varies a little with age. It boils at 356° F., and dissolves in 13 parts of water, but more readily in alcohol and ether. In the air it is rapidly converted into benzoic acid by the absorption of oxygen. It has to be carefully protected from air and light and kept in well-closed bottles in a dark place. The crude oil, containing from 2 to 5 per cent. prussic acid, has generally a yellowish color.
Oil of bitter almonds may be prepared artificially in many ways. By allowing chlorine to flow into boiling toluene, the latter is converted into benzyl chloride:-
C
6H
5(CH
3)
+ Cl
2=
C
6H
5(CH
2Cl)
+ HCl
toluene chlorine benzyl chloride hydrogen chlorideBy withdrawing the chlorine and one atom hydrogen from the benzyl chloride and introducing for it one atom oxygen, the benzyl chloride is converted into benzaldehyde. This conversion is readily effected by continuously boiling, best with the introduction of carbonic acid, 1 part of benzyl chloride with 1½ parts of lead nitrate and 10 parts of water, and finally distilling the benzaldehyde off by steam. The decomposition takes place according to the following equation:—
2[C6H5(CH2Cl)] + Pb(NO3)2 =
2[C6H5(CHO)] + PbCl2 + N2O3 + H2O.
The crude benzaldehyde thus obtained is agitated with warm solution of acid sodium sulphite, the solution formed thereby is separated from undissolved oily particles and cooled, whereby a combination of benzaldehyde with acid sodium sulphate crystallizes out. This combination is separated from the remaining fluid, decomposed by acid and submitted to distillation, whereby benzaldehyde passes over. Large quantities of benzaldehyde are at present prepared according to this method. The identity of benzaldehyde with oil of bitter almonds has been established by Lippmann and Hawliczek.
Genuine oil of almonds is much adulterated, chiefly with alcohol, nitrobenzole, and various cheaper oils. An addition of 3 to 5 per cent. of alcohol is frequently made by Italian dealers in order to conceal a content of water, which at a low temperature is apt to render the oil turbid. To detect the presence of alcohol, moderately heat a sample of the oil in a distilling apparatus and compound the drops, first passing over with sodium carbonate solution and then with potassium iodide solution. In the presence of alcohol a yellowish crystalline precipitate of iodoform is formed.
An addition of synthetically composed oil might seem of no importance, since the natural oil does not differ from it. However, for very fine perfumery the natural oil cannot be replaced by the artificial, it having been thus far impossible to obtain the latter absolutely chemically pure. It always contains small quantities of undecomposed chlorine combinations which injure the taste and odor. To detect such oil in the natural oil, bring a few drops upon a tuft of cotton and ignite it. Over the burning flame invert a beaker moistened inside with water. On the moist sides of the beaker the soot and hydrochloric acid formed by the combustion of the chlorine combination are precipitated. When the flame is extinguished, the beaker is rinsed out with water, the fluid filtered and tested for chlorine with nitrate of silver. An addition of 10 per cent. artificial oil can in this manner be accurately determined.
If genuine oil of bitter almonds containing prussic acid, be heated with an excess of alcoholic potash lye, and the excess of the latter be neutralized with hydrochloric acid, benzoin amounting to 40 to 50 per cent. of the weight of oil of bitter almonds is, according to A. Kremel, separated. By subjecting artificial oil of bitter almonds to the same treatment, no benzoin is separated, so that the genuine oil can in this manner be distinguished from the artificial. Kremel further found that oil of bitter almonds prepared from apricot kernels, when treated in an analogous manner, yielded considerably less benzoin, and that cherry-laurel oil containing prussic acid, which has been considered identical with oil of bitter almonds, separated no benzoin whatever. Should further experiments prove the constancy of this phenomenon, this reaction would be a convenient means of distinguishing the four products.
An adulteration with nitrobenzole and other volatile oils is recognized by mixing 2 drops of the oil with 100 drops of distilled water, and shaking vigorously. Pure oil must completely dissolve. However, the test yields accurate results only with the use of actually pure distilled water and by accurately observing the above-mentioned proportions. If to 5 cubic centimeters of 90 per cent. alcohol and an equal quantity of distilled water in a test-tube, 10 drops of the oil be added, and, after closing the tube with the finger, mixture be effected by gently turning the tube twice upside down, a clear solution will immediately result if the oil is pure. If, however, it contains nitrobenzole, even only 1 per cent., the latter separates, at first rendering the fluid turbid, but in the course of a minute, when gently agitated, it floats in the form of minute drops upon the fluid, while, when at rest, these drops collect to larger ones on the bottom of the test-tube. If the oil becomes only turbid, adulteration with other volatile oils is indicated. Another test, given by Wagner, is based upon the difference in the specific gravity of mixtures of oil of bitter almonds with oil of mirbane. The specific gravity of commercial oil of bitter almonds varies between 1.040 and 1.043 and that of oil of mirbane between 1.180 and 1.201.
5 c. c. of pure oil of bitter almonds weigh 5.29 grammes.
5 " mixed with ¼ oil of mirbane " 5.39 "
5 " " " ½ " " " 5.57 "
5 " " " ¾ " " " 5.75 "
5 " of pure " " " 5.90 "
Oil of bitter almonds is much used in the fabrication of perfumery. In a pure state its odor is by no means agreeable, but rather strong and stupefying. When strongly diluted it is, however, very pleasant.
Angelica oil is obtained by distillation with water from the root of Angelica Archangelica L., natural order Umbelliferae. The oil is lighter than water, possesses the spicy odor of the root and an aromatic pungent taste. It consists mostly of a terpene which turns the plane of polarization to the right, and boils at 320° F.
Besides the oil from the root, one obtained from the seeds also occurs in commerce. It is, however, more expensive. In a fresh state it is amber-yellow, and has a specific gravity of 0.8549 at 59° F.; older oil is thickly-fluid, brown, and has a specific gravity of O.9086. It contains a terpene which turns the plane of polarization to the right, and has a lemon-like odor. It is used for fine perfumery.
Anise-seed oil (oleum anisi). The anise (Pimpinella anisum L.), natural order Umbelliferae, contains volatile oil in all parts, but chiefly in the seeds. Dry anise-seed yields by distillation 2½ to 3 per cent. of oil, while the peduncle and chaff contain at the utmost 1 per cent. of oil, which is said to be richer in stearoptene. The anise-seed oil prepared in Southern Russia has always been highly valued, but as it is generally considerably adulterated, the Leipsic manufacturers of volatile oils prefer to import the seed and distill it themselves.
Freshly prepared anise-seed oil is colorless or straw-yellow, has the odor of anise and a sweetish taste, leaving a burning sensation upon the tongue. It is thinly fluid at 68° F., but commences to congeal at a somewhat lower temperature, and the sooner the more stearoptene it contains. Good oil should become solid at from 57.2° to 60.8° F. It has a specific gravity of 0.980 to 0.995 at 59° F. The specific gravity varies with the content of stearoptene; the greater the latter the higher the specific gravity. Good anise-seed oil contains 5 to 10 per cent. of terpene and 90 to 95 per cent. of a stearoptene, called anethol, C10H12O, on which the value of the oil depends. The anethol can be separated from the oil by cooling to 32° F., and forms colorless crystals. It has an agreeable odor and intensely sweet taste, is sparingly soluble in water, but readily in alcohol, ether, and other solvents of volatile oils. Good anethol has a specific gravity of 0.986, and melts at 69° to 70° F. By frequent contact with the air a small portion of the anethol is oxidized, very likely to anisaldehyde. By this process the specific gravity is raised and the melting point lowered.
Anise-seed oil is soluble in 5 parts of 90 per cent. alcohol, and with 3½ times its volume of petroleum-ether yields a clear mixture. Its mixture with four times its weight of petroleum-ether is turbid, but becomes clear in ten minutes, while that with five times its volume of petroleum-ether remains for a longer time turbid. In a fluid state the oil, when exposed to the air, becomes resinous and loses its property to crystallize. It should, therefore, be kept in tightly-closed bottles in a cool, shady place.
Anise-seed oil is used in perfuming soaps and mouth waters. It should, however, be used with prudence, since the sweetish, penetrating odor of the oil readily overcomes the other volatile oils in the mixture, and renders them inoperative.
Star anise oil very much resembles the ordinary anise-seed oil. It is obtained from star anise, the fruit of Illicium anisatum, a tree formerly supposed to be indigenous to Cochin China, and cultivated in China, Japan, and the Phillipine Islands. However, according to Messrs. Bourgeoin-Meiffre, a French firm of Hanoï (Tonkin), the star anise oil found in commerce is exclusively produced in the French colony Tonkin (Province Langson), the French government having made over the entire sale of the oil to the above-mentioned firm.
According to a memoir published by Dr. Blondel, of Paris, the star anise tree is not indigenous to the Chinese provinces Yunnan, Quang-si, and Fo-Rien, but to the province Langson, which has by conquest passed into French possession. Hence, the producers of star anise and star anise oil are now under French control and, as it seems, are obliged to sell all the oil produced to the above-mentioned firm. If these statements should prove correct, the Chinese harbors Macao and Hong Kong, from which the greater portion of star anise oil was formerly exported, will lose their importance in this respect and the product find its way direct from Hanoï via Hayphong to Marseilles. The first shipment from Bourgeoin-Meiffre arrived in Europe in December, 1890. According to Messrs. Schimmel & Co.'s report, the product is put up and packed exactly like that formerly shipped from Hong Kong, and the oil of excellent quality.
Star anise oil differs from the ordinary oil in containing a much smaller quantity of anethol, and hence congealing only at a temperature of from 41° to 50° F. Besides the odor of the terpene contained in star anise oil differs from that of the ordinary oil. Admixtures of star anise oil can, therefore, be generally recognized by the odor. Other methods recommended for its detection are unreliable.
Balm oil.—The leaves of this plant, Melissa officinalis, yield by distillation a volatile oil sometimes called oil of melissa. It is colorless or yellowish, of a pleasant odor, has a specific gravity of 0.85 to 0.92, shows a slightly acid reaction and dissolves in 2 to 3 parts of alcohol. It must not be confounded with the so-called East India oil of melissa or citronella oil from Andropogon Nardus L. Balm oil is occasionally used in the preparation of eau de Cologne.
Basil oil is distilled in Southern France from the fresh leaves of Ocymum basilicum L., natural order Labiatæ. The oil shows the peculiar odor of the herb and crystallizes a few degrees above 32° F. In perfumery it is used as an addition to violet and other preparations. The French also prepare a pommade basilique, which serves as a cheap substitute for violet pomade.
Bayberry oil, or oil of bay leaves, is extracted by distillation from the leaves of Myrcia acris or the bayberry tree. Many varieties of the tree exist throughout the West Indies, which are scarcely to be distinguished botanically, but have quite a different odor from that of the genuine tree. Great care must, therefore, be taken in the collection of the leaves which are to be used, as the admixture of a small quantity of the other leaves may entirely spoil the product of distillation. Two oils are obtained, a light oil of specific gravity of 0.870 to 0.990, and a heavy oil with specific gravity 1.023 to 1.037. When first distilled the oil is colorless, but by exposure to the air quickly acquires a yellowish tint and, if the exposure be continued, becomes quite dark in color. The odor of the freshly-distilled oil is rank, but in the course of from three to six months it becomes mellow, and ripens into the agreeable fragrance so much liked in the best specimens of bay-rum. The oil is soluble in all proportions in 95 per cent. alcohol, also in ether and petroleum benzine. Its chief use is for the preparation of bay-rum.
Bergamot oil is obtained from the rinds of the fruit of citrus bergamia, a tree belonging to the natural order Aurantiaceæ. The rind is grated and the oil running off separated from the aqueous fluid and cellular substance by means of a separating funnel, or the grated mass is distilled in a current of carbonic acid. The oil is very fluid and pale yellow, but poorer qualities are frequently greenish or brownish. When distilled with water it becomes perfectly colorless, but is less durable. Its odor is very pleasant, somewhat like a mixture of orange and lemon oils. Its specific gravity is 0.87 to 0.89. By standing for some time, the oil separates white crystalline scales (stearoptene), which melt at 223° F. The oil becomes solid a few degrees below the freezing point. The Messina oil of bergamot is considered the best. From other volatile oils of the orange family, bergamot oil differs in dissolving readily in caustic potash, forming a clear solution. It has, however, the same property as other oils of a similar origin, of igniting with iodine and not dissolving santalin, the red resinous coloring matter of santal-wood.
Bergamot oil may be tested as to its purity by mixing it with alcohol. It becomes pale gray-yellow, forms a sediment which adheres firmly to the vessel and, on shaking, floats about in the form of flakes. After two days the sediment is inconsiderable and difficult to divide into flakes in the clear yellow fluid by shaking. The oil is frequently adulterated with alcohol. To detect such adulteration, Righini recommends the following method: Mix 15 parts of the oil with a like quantity of pure olive oil or oil of sweet almonds. If alcohol is present, it immediately separates, like water, from the fat oil; if no separation takes place the oil is not adulterated with alcohol. The tannin test also gives reliable results. In storing oil of bergamot great care must be exercised to exclude air and light, as it is one of the most changeable oils and soon acquires an odor resembling that of turpentine.
Large quantities of oil of bergamot are used in perfumery. It forms, so to say, the basis for most of the finer products. In Cologne water it forms the principal constituent in the mixture of volatile oils.
Cajeput oil (oleum cajeputi).—This oil is obtained by distillation from the leaves of several species of Melaleucæ, natural order Caryophyllaceæ, indigenous to the East Indies, Banda, and Malabar. The ordinary oil has a greenish color and possesses a strong odor of camphor and a pungent taste. It is chiefly imported by way of Amsterdam, where it is partially discolored by rectification, so that two kinds, the white and green cajeput oil, are brought into commerce. The color of the latter is generally supposed to be due to a resinous substance containing chlorophyl, though others assert that it originates from the copper of the distilling apparatus and the copper flasks in which it is dispatched. The specific gravity of the oil varies between 0.910 and 0.940, though specifically lighter and heavier oils are said to occur.
It is claimed that an artificial cajeput oil is often prepared from camphor and rosemary oil, the green color being obtained by distillation with milfoil. The presence of camphor may be readily determined by thoroughly triturating a few drops of the oil with sugar and then dissolving in water, whereby the particles of camphor separate in the form of white flakes upon the surface.
Cajeput oil is frequently adulterated with oil of turpentine and rosemary oil. Such adulteration is recognized by pure cajeput oil dissolving clear in equal parts of 90 per cent. alcohol, which is not the case with the other two oils.
Camomile or chamomile oil (oleum anthemidis).—Two varieties of oil of camomile are found in commerce, one green and the other blue. The first is derived from the flowers of the genuine or Roman camomile (Anthemis nobilis) and the blue from the common variety (Matricaria chamomila). The last oil is the one chiefly used in the manufacture of perfumery and in medicine.
Blue camomile oil is generally obtained by distillation. In distilling, metal Florentine flasks should be used, as the oil adheres tenaciously to glass vessels and the distillate has to be treated with ether. The pure oil has a beautiful blue color, and on heating forms blue vapors. It has a penetrating odor which only by strong dilution becomes similar to that of camomile. By storing in the light and the simultaneous presence of air, the oil turns green; later on, brown, and is finally converted into a thickly-fluid, brownish mass.
Green camomile oil from the genuine or Roman camomile possesses an agreeable odor of fresh lemons; it is more seldom used than the other.
On account of the slight yield obtained from the flowers, camomile oil is rather expensive.
Caraway oil (oleum carui) is obtained by distillation from the seeds of the well-known aromatic plant Carum carui, or the caraway, natural order Umbelliferæ. In a fresh, purified state the oil is colorless, very thinly-fluid and possesses a pungent taste. The oil prepared from cleansed Dutch seed is best liked, while that distilled from Norwegian or Tyrolese seed is not much in demand, its taste and odor not being so pure on account of the many impurities mixed with these kinds of seed.
Caraway oil consists mainly of a terpene, C10H16, called carvene, specific gravity 0.870, and of carvol, specific gravity 0.960. The richer the oil in carvol, the higher its specific gravity. Good caraway oil should have a specific gravity of 0.900 to 0.910. The carvol being the actual bearer of the aroma, the value of the oil exclusively depends on the content of it. In the better varieties of oil, the content of carvol amounts to from 45 to 50 per cent., while poorer qualities generally contain only from 40 to 42 per cent. The carvol and carvene are now frequently separated by fractional distillation. The carvol, which has three times as strong an odor and taste as the carvene, dissolves with much greater facility in alcohol. The carvene being offered at very low prices might be suitable for perfuming cheap soaps.
Caraway oil obtained by distillation from the plant has a less agreeable odor than that from the seed, and possesses an acrid resinous taste.
The purity of caraway oil is recognized by its dissolving clear in equal parts of 90 per cent. alcohol. If such is not the case, the oil contains either an admixture of oil of turpentine or does not possess the full normal content of carvol. Pure caraway oil does not detonate with iodine, which is the case with oil containing oil of turpentine.
Caraway oil is chiefly used for perfuming soap; for handkerchief perfumes it is not suitable.
Cedar oil (oleum cedri) is obtained by distillation from the shavings of the wood of the American or Virginia cedar (Juniperus virginiana). For the distillation of oil the waste falling off in the manufacture of lead-pencils is almost exclusively used. It yields about 2 to 3 per cent. of oil. The oil is thinly-fluid, of specific gravity 0.9622, of a greenish color, and an agreeable but not very penetrating odor. It is a mixture of a terpene, boiling at about 540° F., and of a hydrocarbon. The latter, which is called cidrin, forms the fluid portion of the oil. It has a specific gravity of 0.984, and boils at about 459° F.
Cedar oil is extensively used in the manufacture of toilet soap, it serving as the basis for other perfumes. Care must, however, be taken that its odor does not preponderate, as in such case it readily produces an unpleasant effect. The oil being cheap, adulteration is scarcely to be feared.
A volatile oil is also obtained by distillation from the leaves of the Juniperus virginiana. In odor it resembles savin oil, and is unfit for perfuming purposes.
Cherry-laurel oil (oleum laurocerasi) is the volatile oil, which contains prussic acid, obtained from the leaves of the cherry-laurel (Prunus laurocerasus, L.). Like bitter almonds, the leaves contain some amygdalin. Hence they are macerated with water and allowed to stand in a warm place for 24 hours. By subsequent distillation a volatile oil is obtained which closely resembles oil of bitter almonds, but differs in some respects. It is colorless or yellowish, rarely reddish, and of specific gravity 1.05 to 1.06. In its behavior towards air, solvents, and reagents, it does not essentially differ from oil of bitter almonds.[5]
To detect oil of mirbane in cherry-laurel oil, Enrico Pega adds some alcohol to the oil to be tested and then mixes it with some alcoholic potash lye and a few drops of ferric chloride solution. After standing for a few hours the mixture is shaken and distilled. A small portion of the oil distilling over is freed from water, poured upon a few small pieces of pure caustic potash in a test-tube, and heated over a lamp. If the sample is pure it remains colorless; in the presence of oil of mirbane it acquires a dark coloration in consequence of the formation of nitrobenzide and aniline, a few drops of calcium chloride solution brought into the mixture producing, for this reason, a violet coloration.
Cherry laurel oil is but seldom used for perfuming purposes.
Cinnamon oils.—There are four different kinds of this oil, viz., Ceylon cinnamon oil, cassia oil, cinnamon root oil, and oil of cinnamon leaves. Though the first two are very much alike, the Ceylon oil is considered the best.
Ceylon cinnamon oil (oleum cinnamoni ceylonici).—Formerly this oil was exclusively distilled from chips and waste of the genuine cinnamon bark of the Cinnamonum ceylonicum, Nees, and came into commerce from Ceylon. However, the fabrication of the oil from cinnamon waste or chips is now extensively carried on in Germany, and this oil, being prepared with the assistance of more perfect apparatus, has almost entirely supplanted that exported from Ceylon.
When fresh, the Ceylon oil is colorless, but when stored for some time it becomes first golden yellow and later on brownish. It is thickly-fluid and heavier than water, its specific gravity being 1.060 to 1.090. It has an agreeable, aromatic odor and a biting but pure, sweet taste. Its principal constituent is cinnamaldehyde (C9H8O), and it contains, besides, 4 to 8 per cent. of eugenol. The presence of the latter in cinnamon oil may be established by shaking with 15 per cent. soda-solution, whereby the eugenol is dissolved, and decomposing the aqueous solution with hydrochloric acid. The eugenol separated thereby gives in alcoholic solution, when compounded with a trace of ferric chloride, a beautiful blue color.
Cassia oil (oleum cassiæ).—In China and Cochin China this oil is obtained by distillation from the bark, unripe fruits, buds, and other waste of the Cinnamonum cassia or Cinnamonum aromaticum, Nees, a tree indigenous to those countries. It has a pale yellow color, which in time becomes brown. It is thickly-fluid, of specific gravity 1.05 to 1.07, and possesses a sweet taste with an acrid after-taste. Like cinnamon oil, it consists chiefly of cinnamaldehyde, but contains no eugenol, and hence can be readily distinguished from Ceylon oil by the above-mentioned reaction. One part of pure cassia oil dissolves in two parts of 80 per cent. alcohol.
Cinnamon root oil and oil of cinnamon leaves.—Neither of these oils contains cinnamaldehyde, but abundant quantities of eugenol, the root oil as much as 50 to 70 per cent. The root oil is quite limpid and has an agreeable odor of cinnamon and cloves. The leaf oil is thickly-fluid, of the consistency of castor oil.
The Ceylon oil is frequently adulterated with cassia oil. Such adulteration is very difficult to detect, and can only be recognized by experts by the odor and taste.
The quality of cassia oil is recognized by the taste and odor, especially on heating, and the high specific gravity, in consequence of which the oil sinks in water. According to Hager, cassia oil is frequently adulterated with oil of cloves. This is, however, scarcely probable, the price of oil of cloves being, on an average, higher than that of cassia oil. The latter, however, is frequently adulterated with cheaper thickly-fluid volatile oils, especially with cedar oil. In this case the oil does not dissolve in the above-mentioned proportion in alcohol.
The value of cassia oil is dependent on its contents of cinnamaldehyde. Hence, the establishment of its actual value requires a quantitative determination of its contents of cinnamaldehyde, which unfortunately presents great difficulties. For this purpose Schimmel & Co. proceed indirectly as follows: 75 grammes of cassia oil in a capacious boiling flask are mixed with 300 grammes of a boiling-hot 30 per cent. solution of acid sodium sulphite, whereby cinnamaldehyde-sodium sulphite is immediately separated. The whole is then vigorously agitated and allowed to rest for a short time. (With oils rich in aldehyde considerable heating generally takes place, which must eventually be moderated by the addition of cold water.) Next add about 200 grammes of hot water and heat the whole, with frequent shaking, in the water-bath until the combination of the aldehyde with the acid-sodium sulphite is completely dissolved, and the non-aldehydes in the form of an oily layer float upon the solution of the aldehyde salt. Now allow the whole to cool, then shake twice with ether; first, with about 200 cubic centimeters, and then with 100; combine the ethereal extracts of the non-aldehydes separated by means of a separatory funnel, and filter them into a capacious, previously-weighed beaker provided with a platinum wire, the lower end of which is bent in the form of a spiral. Now evaporate the ether as much as possible, by placing the beaker in hot water. When by swinging the beaker the remaining fluid no longer foams up, allow to cool off and weigh. Now return the beaker-glass to the water-bath for ten minutes, weigh again after cooling, and repeat the operation until the difference between two weighings does not amount to more than 0.3 gramme at the utmost. The weighing previous to the last is taken as the correct one.[6]
The weight of the non-aldehydes thus obtained is deducted from the cassia oil used, the difference giving the content of cinnamaldehyde in the latter.
For example:—
Used 79.71 grammes of oil.
First weighing of the beaker after evaporating the ether
147.55
grammes
Second
146.84
"
Third
146.58
"
Tare of the beaker
128.34
"
Hence non-aldehydes in the oil
146.84 grammes.
Less tare
128.34 "
———
= 18.50 grammes.
Calculated to per cent., 23.1 per cent.
100 - 23.1 = 76.9 per cent. cinnamaldehyde.
By accurately following the directions given, the difference between two controlling determinations will be only a few tenths per cent., seldom as much as 1 per cent. For practical purposes, for which alone this method is intended, this is more than sufficient.
According to the reports of Schimmel & Co., all the cassia oil brought into commerce from China was for a considerable time adulterated with resin and petroleum, they having found as much as 30 per cent. of resin in the oil. Such adulteration can be established by the determination of the specific gravity and distilling the oil. Good cassia oil should show a specific gravity of 1.05 to 1.07 at 59° F., and by distillation 90 per cent. of pure cassia oil must pass over. The residue should not solidify after cooling and acquire the character of a brittle resin; it must remain at least thickly-fluid, and under no conditions amount to more than 10 per cent.
Citron oil (oleum citri), from the peel of the fruit of Citrus medica or the citron tree. The oil is prepared in a similar manner to that of oil of bergamot, either by expression or distillation, the latter process yielding more and purer oil.
Rectified citron oil is colorless, of an agreeable penetrating odor and acrid taste, and very sensitive to light and air. By exposure to light it turns yellow, and if air be admitted at the same time, it is first converted into a fluid which, on account of its content of ozone, possesses strong bleaching powers. The oil at the same time acquires a disagreeable odor, resembling that of oil of turpentine, and is finally converted into a resinous mass.
Citron oil is frequently adulterated with oil of orange and sometimes with oil of bergamot. These adulterations are readily detected by an experienced person by the odor, this being in fact the best guide. The specific gravity of citron oil is 0.850 at 59° F.; it boils at from 332.6° to 343.4° F. and congeals at 4° F.
Citronella oil (oleum citronellæ) is chiefly distilled in Ceylon from the lemon grass, Andropogon Nardus, L. It is quite limpid, of a greenish-yellow to brown color, and has an odor resembling that of genuine citron oil. Its specific gravity is 0.896 at 59° F., and it boils at from 392° to 410° F. Of the various oils reaching the market that with the trade-mark "Fisher" is most in demand, it being distinguished by special purity. Edward Kremers has found in citronella oil an aldehyde, C7H14O, a terpene, C10H16, citronellol, which is isomeric with borneol; further, acetic acid and valerianic acid.
The Indian distillers, it is claimed, adulterate the citronella oil with petroleum, an addition up to 25 per cent. being not uncommon.
According to experiments by Schimmel & Co., pure citronella oil must give a clear solution, when 1 part of the oil is vigorously shaken with 10 parts of 80 per cent. alcohol. If, in executing the test, the kind of turbidity is observed, and whether the portion insoluble in alcohol separates, after standing, upon the surface or on the bottom of the fluid, and further, if the above-mentioned quantity of alcohol is not added at one time, but at first only 1 or 2 parts of it, a conclusion may be drawn as to the kind and quantity of the adulterant.
Petroleum causes a milky-white turbidity, while in the presence of fat oil the mixture becomes turbid, but not actually milky. As a rule, fat oil deposits, after standing, on the bottom, while petroleum floats upon the surface of the fluid. Citronella oil adulterated with fat oil does not dissolve in 1 to 2 parts nor in 10 parts of 80 per cent. alcohol, while oil adulterated with not too large a quantity of petroleum, gives a clear solution with 1 to 2 parts. The determination of the specific gravity may also serve for the detection of adulterations. This holds good, however, only for petroleum, which reduces the specific gravity, an addition of fat oil producing no deviation in this respect. The specific gravity of the oil should not be below 0.895 at 59° F.
Citronella oil is much used for perfuming cheap hair oils and toilet soaps; it is the chief constituent of all perfumes for honey-soaps. In the American soap industry it is extensively used, the yearly consumption being estimated at 1½ million ounces.
Cloves, oil of (oleum caryophylli), is obtained by distillation with steam, or by extraction from the cloves of commerce, which are the dried unexpanded flower buds of Caryophyllus aromaticus, L., or the clove tree. Oil of cloves, when fresh, is almost colorless, but on exposure to air acquires a brownish coloration and a thickly fluid consistency. It has the aromatic taste and odor of cloves, and a specific gravity of 1.300 to 1.065. It frequently shows a slightly acid reaction, boils at 482° F., and congeals at 4° F. It is readily soluble in alcohol, ether, and strong acetic acid. It consists of a terpene (C10H16) and eugenol (C10H12O2), the odor of the oil being due to the latter. The terpene has a specific gravity of 0.918, and in distilling passes over first (light oil of cloves). The eugenol, when fresh, is colorless, has the odor and taste of cloves, a specific gravity of 1.063 at 65° F., boils at 487.4° F., is insoluble in water and glycerin, but soluble in alcohol, ether and glacial acetic acid. Its alcoholic solution is colored magnificently blue by ferric chloride. If in an alkaline solution it is oxidized with potassium permanganate, vanillin being formed.
An inferior quality of oil is obtained from the stems of the clove buds. It dissolves with greater difficulty than the oil prepared from the buds, and has a darker red-brown color.
To test the value of oil of cloves, introduce, according to Stohman, into a graduated glass cylinder 10 volumes ether, 10 oil of cloves, and 30 of a 10-per cent. soda solution. After vigorous shaking, the eugenol dissolves; the increase in volume of the aqueous fluid is then proportional to the quantity of eugenol present. For more exact determinations, dissolve a weighed quantity of oil, repeatedly shake the aqueous fluid with ether to remove the terpene, then decompose the eugenol-sodium with dilute sulphuric acid, dissolve the separated eugenol in ether and weigh after evaporating the ethereal fluid. Good oil of cloves does not contain less than 80 per cent. of eugenol, and frequently 90 per cent. or more.
Oil of cloves is chiefly adulterated with copaiba oil and cedar oil. Such adulteration is recognized by the oil not forming a clear solution in every proportion with alcohol, as is the case with pure oil of cloves.
Oil of cloves is much used for perfuming purposes.
Eucalyptus oil (oleum eucalypti) is obtained from the leaves of various trees of the eucalyptus family. According to Merk two kinds of oil must be strictly kept apart: oleum eucalypti from the leaves of eucalyptus globulus and oleum eucalypti australe, the former being used in medicine, and the latter, which is considerably cheaper, chiefly for perfuming purposes. However, Piesse's opinion that eucalyptus oil, as far as its odor is concerned, does not deserve to be classed among perfumes is undoubtedly correct. It has an odor between that of oil of turpentine and cajeput oil, and as long as perfumery is the art of sweet odors, such oil cannot be designated a perfume.
When not rectified, eucalyptus oil is mostly yellowish or bluish. In a rectified state it is colorless, clear, limpid, lighter than water, of a strong odor, and acrid taste. The oil from eucalyptus globulus has a specific gravity of 0.900 to 0.925, and dissolves in every proportion in 90 per cent. alcohol. It is optically inactive or turns the plane of polarization slightly to the right. On standing with sodium it acquires a yellowish coloration, and does not detonate with iodine. The oil from eucalyptus australe has a specific gravity of 0.86 to 0.87, and is but sparingly soluble in 90 per cent. alcohol, so that even a solution prepared in the proportion of 1:15 is turbid. It turns the plane of polarization strongly to the left; acquires, on standing with sodium, a red coloration, and detonates with iodine.
Eucalyptus oil consists of eucalyptol and eucalyptene, and perhaps other hydrocarbons. The content of the first, on which depends the medicinal value of the oil, varies very much in the oils from the different species of eucalyptus, the oil from some species, it is said, containing no eucalyptol whatever.
Eucalyptol (C24H20O2) is limpid, colorless, turns the plane of polarization, has a specific gravity of 0.905, and boils at 347° F. Its vapor mixed with air has an agreeable, refreshing taste, and its dilute solutions remind one of roses. Eucalyptene (C24H18) has a specific gravity of 0.836, and boils at 329° F.
Fennel oil (oleum fœniculi) is derived by distillation from the fruits of Fœniculum vulgare, Gaertner. Large quantities of it are produced in Saxony, and also in Galicea. It is quite colorless, limpid, of specific gravity 0.940 to 0.970 and, with a full content of stearoptene, possesses a nauseous sweet taste and odor. It contains 60 to 70 per cent. of anethol and congeals at from 41° to 50° F. to a crystalline mass. The leaves of the plant also contain a volatile oil, which is, however, less valued than the seed-oil.
Good fennel oil should dissolve clear in 1 to 2 parts of 90 per cent. alcohol. Direct adulterations of this oil do not occur, but the stearoptene is frequently withdrawn by fractional distillation whereby the oil loses much in value. Such oil freed from stearoptene does not congeal, has a more bitter than sweet taste and does not dissolve in the above-mentioned proportion in alcohol.
In perfumery fennel oil is but little used; sometimes in connection with other volatile oils for perfuming soaps.
Geranium oil, palmarosa oil, Turkish geranium oil is obtained from Andropogon Pachnodes. It is yellowish, limpid, of specific gravity 0.890 at 59° F., possesses a very agreeable rose-like odor resembling that of geranium oils from Pelargonium radula, Aiton, and for this reason is generally designated as Turkish geranium oil. The odor of the oil is improved by shaking it with water containing lemon juice, any content of copper being thereby removed. The washed oil is then brought into shallow dishes and exposed for two or three weeks to the sun, whereby its odor becomes still more like that of rose oil. The oil thus prepared is much used for adulterating rose oil. Turkish geranium oil is also much used for the adulteration of genuine geranium oil and is itself adulterated with oil of turpentine. It is extensively employed in perfumery, especially for perfuming hair oils and pomades, and in conjunction with geranium oils for rose soap.
East Indian geranium oil is obtained, chiefly in the Presidency of Bombay, from Andropogon Schoenantus, L. It is greenish-yellow to yellow-brown, has a specific gravity of 0.906 at 59° F., and consists mainly of geraniol (C10H18O). Its odor is rose-like, though modified by a lemon-like odor. It is principally used for perfuming cheaper articles.
French and African geranium oils (oleum geranii) are obtained by distillation with water from the leaves of various species of pelargonium. Many different kinds of this oil are found in commerce. The finest and most expensive are the Spanish and French geranium oils, so-called rosé, which are distinguished by their fine odor, closely resembling that of rose oil. They are derived from Pelargonium radula, and are either yellowish, brownish, or pale green, the brownish oils being preferred. It congeals at 60.8° F. and turns the plane of polarization to the right. Another good geranium oil is the African, which is chiefly prepared in Algiers from Pelargonium roseum, Wildenow, and P. odoratissimum, Aiton. It closely resembles the French oil, but turns the plane of polarization to the left.
French geranium oil is said to be frequently adulterated with fat or copaiba oil; but geranium oil being soluble in 70 per cent. alcohol, such adulterations are readily detected. Add to 5 cubic centimeters of 70 per cent. alcohol (specific gravity 0.890) at 59° to 62.5° F., 10 drops of the oil, and shake. If a clear solution results the oil is very likely unadulterated.
The so-called Turkish geranium oil is frequently found mixed with cocoanut oil. To detect this, place the oil in a test-tube in ice or a cold mixture for several hours, whereby the cocoanut oil separates as a white substance. Adulterations of upward to 20 per cent. are said to frequently occur.
Reliable tests to detect an adulteration of the better qualities of geranium oil with those of a lower grade do not exist, the odor being the only guide.
Jasmine oil or oil of jessamine, from the flowers of Jasminium officinale, L., and J. grandiflorum.—The oil is exclusively obtained by the absorption process, and is the most prized by the perfumer. It is, however, exceedingly rare on account of the enormous cost of its production. The extract of jasmine, the "essence de jasmine" of the French manufactories, is a solution of the oil, as obtained by extraction with lard or beef suet, in strong spirit of wine. The odor of jasmine oil is so peculiar that it is without comparison, and as such cannot be imitated.
Juniper oil (oleum juniperi) is obtained by distillation from the fruits of Juniperus communis, L., or juniper. The berries used for the purpose should be fully grown and fresh and bruised before being placed in the still. Unripe berries yield a smaller quantity and an oil of somewhat different properties than ripe berries. The oil obtained by distillation with steam is colorless and that by ordinary distillation yellowish, the former having a specific gravity of 0.840 to 0.860 and the latter of 0.850 to 0.900. It consists mainly of terpenes. By standing, a stearoptene is separated, which crystallizes in feathery needles from hot spirit of wine. The odor and taste of juniper oil remind one at the same time of juniper berries and oil of turpentine.
Juniper oil has a great tendency to thicken; it becomes resinous, acid and thickly fluid, formic acid being formed. It should be kept in well-closed bottles, and protected from light. It is frequently adulterated with oil of turpentine and juniper-wood oil. It may be tested by its behavior towards alcohol, as well as by the taste. A drop of the oil rubbed up with sugar, and shaken with 500 grammes of water, should not impart an acrid taste to the water. Juniper oil gives a clear solution with ½ part absolute alcohol; by a larger quantity it is rendered turbid.
Lavender oil (oleum lavandulæ.)—Large quantities of this oil are distilled in Southern France, in the neighborhood of Grasse and Nimes, from the flowers of lavandula officinalis, Chaix, which grows wild in that region. It is limpid, colorless, or yellowish, has a strong odor and a pungent, aromatic, somewhat bitter taste. With 90 per cent. alcohol it mixes clear in every proportion, boils at 320° F., and has a specific gravity of 0.876 to 0.905. It turns the plane of polarization to the left.
The best French lavender oil, distilled from pure flowers only, is brought into commerce under the name, "Essence de Lavande Montblanc." It is distinguished from all other kinds, in the preparation of which more or less stems and leaves are used, by its extremely agreeable odor.
Lavender is also extensively cultivated in Mitchan and Hitchin, England, and used for the preparation of an especially fine oil, the odor of which surpasses even that of the best French product. It is, however, comparatively expensive.
From the leaves and flowers of lavandula spica the spike oil is obtained by distillation. It is colorless, or yellow, and in odor approaches rosemary oil more than lavender oil. Its boiling point, like that of lavender oil, is at 366.8° F., and its specific gravity 0.96. Spike oil turns the plane of polarization only slightly to the left, the deviation scarcely ever exceeding 0.8°.
Lavender oils are very sensitive to light and air, they becoming ozonized under their influence, and acquire an odor like turpentine. Hence they must be kept in well-closed vessels in a dark place.
Oil of lavender is frequently adulterated, chiefly with alcohol, fat oils, oil of turpentine, and spike oil. To test the oil, mix a drop of it with 10 cubic centimeters of warm water, and test the odor, which should be pure and agreeably lavender-like. The taste of the vigorously agitated water should be transiently bitter aromatic. One volume of the oil should give a turbid mixture with one volume of dilute alcohol (specific gravity 0.895), but a perfectly clear one with three volumes. On shaking 0.5 cubic centimeter of the oil with a few grains of rosaniline it remains uncolored, but, in the presence of even a trace of alcohol, it acquires a red coloration. By mixing in a graduated cylinder equal volumes of the oil and distilled water, and shaking vigorously, the oil, after the water has settled, shows a decrease in volume if alcohol be present. The presence of fat oil can be readily recognized by bringing a drop of the oil to be tested upon filtering paper; a grease stain is formed, which disappears neither at the ordinary temperature nor by heating. Adulteration with oil of turpentine is recognized by the boiling point, that of oil of turpentine being 312.8° F., and that of oil of lavender, as previously stated, 366.8° F.
Spike oil should mix clear with equal parts of 90 per cent. alcohol; the contrary would indicate adulteration with oil of turpentine.
For perfumery, lavender oil is of great importance, it being much employed by itself, as well as mixed with other oils.
Lemon oil (oleum limonis) is obtained by various processes from the rinds of lemons. The best and most delicately-scented oil is obtained by the so-called sponge process in use in Southern Italy and Sicily. The rinds are soaked from fifteen to twenty-five minutes in water, to which sometimes a little soda is added. They are taken up singly in the right hand and the outer surface of each is firmly pressed against a large and rather hard-grained sponge held in the left hand and secured by a strap. Two or three sharp turns of the wrist impart what may be called a screw-pressure to the rind, thus effectually fracturing the oil cells, the sponge absorbing the contents. The sponge is constantly held over an earthen jar and occasionally squeezed into it. The fluid in the jar quickly separates into three different products—the dregs or deposit of mucilaginous and cellular matter, some fruit juice, and the pure oil, which floats on the top. The latter, when bright and clear, is passed, by means of a small glass siphon, into the cans of thin copper, in which, after sealing, it is stored away for export.
The above described primitive mode of fabrication furnishes the most highly prized oils of commerce; they are called hand-pressed oils or essences preparées a l'éponge. In the same manner are obtained the oils from the sweet and bitter pomegranate, the bergamotte, and mandarin orange.
Another method of expressing the oil is that of the écuelle à piquer, much used in the region about Nice. The oils obtained by this method, which are also of a very fine quality, are marked essence à l'écuelle or au zeste. The apparatus consists of a round shallow pan of copper or brass, having a receptacle for the oil at its lowest part and a lip on one side for pouring, and studded on its concavity by strong blunt spikes. The workman takes the fruit and rolls it gently but quickly around the inside of the écuelle; the spikes prick the oil sacs, whereupon the oil, running down the spikes and the concavity of the pan, collects in the reservoir at the lowest part. The oil is filtered and then poured into clean glass bottles, in which the impurities are allowed to settle.
In Reggio, where especially much bergamot oil is manufactured, sheet-metal bowl-like vessels, studded inside with sharp ribs, are used. Six to eight fruits are placed in the vessel. A movable lid closes the vessel, so that there is just enough space for the fruits between the lid and the bottom of the vessel. If now the lid be revolved by hand-or steam-power, the rinds of the fruit are torn apart, and the oil together with the juice runs through the sieve-bottom of the apparatus into a cylindrical vessel where it clarifies.
The third method of obtaining the oil is by expression. The grated rind is placed in hair mats, and subjected to powerful pressure by means of a screw or lever press.
The process of distillation is carried on as follows: The peels, which should be from select fruit, are sprinkled with powdered salt, and a few hours afterwards sufficient water to moisten them is poured over them. A day or two afterwards more water is added, and the whole is distilled until either no more oil separates or the steam, at first purely fragrant, begins to acquire a rank and rather unpleasant smell. The oil obtained by distillation is inferior to the others.
[5] Compare Kremel's observations, p. 91.
[6] The manner of expelling the ether is of great influence upon the accuracy of the result. Though the non-aldehydes volatilize with difficulty, they are volatile, and hence the ether must be quickly expelled, and the beaker not allowed to stand longer upon the water-bath than necessary for the evaporation of the ether.
